Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus
- PMID: 35852338
- PMCID: PMC9430161
- DOI: 10.1128/spectrum.00250-22
Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus
Abstract
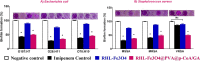
Surface-growing antibiotic-resistant pathogenic bacteria such as Escherichia coli and Staphylococcus aureus are emerging as a global health challenge due to dilemmas in clinical treatment. Furthermore, their pathogenesis, including increasingly serious antimicrobial resistance and biofilm formation, makes them challenging to treat by conventional therapy. Therefore, the development of novel antivirulence strategies will undoubtedly provide a path forward in combatting these resistant bacterial infections. In this regard, we developed novel biosurfactant-coated nanoparticles to combine the antiadhesive/antibiofilm properties of rhamnolipid (RHL)-coated Fe3O4 nanoparticles (NPs) with each of the p-coumaric acid (p-CoA) and gallic acid (GA) antimicrobial drugs by using the most available polymer common coatings (PVA) to expand the range of effective antibacterial drugs, as well as a mechanism for their synergistic effect via a simple method of preparation. Mechanistically, the average size of bare Fe3O4 NPs was ~15 nm, while RHL-coated Fe3O4@PVA@p-CoA/GA was about ~254 nm, with a drop in zeta potential from -18.7 mV to -34.3 mV, which helped increase stability. Our data show that RHL-Fe3O4@PVA@p-CoA/GA biosurfactant NPs can remarkably interfere with bacterial growth and significantly inhibited biofilm formation to more than 50% via downregulating IcaABCD and CsgBAC operons, which are responsible for slime layer formation and curli fimbriae production in S. aureus and E. coli, respectively. The novelty regarding the activity of RHL-Fe3O4@PVA@p-CoA/GA biosurfactant NPs reveals their potential effect as an alternative multitarget antivirulence candidate to minimize infection severity by inhibiting biofilm development. Therefore, they could be used in antibacterial coatings and wound dressings in the future. IMPORTANCE Antimicrobial resistance poses a great threat and challenge to humanity. Therefore, the search for alternative ways to target and eliminate microbes from plant, animal, and marine microorganisms is one of the world's concerns today. Furthermore, the extraordinary capacity of S. aureus and E. coli to resist standard antibacterial drugs is the dilemma of all currently used remedies. Methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) have become widespread, leading to no remedies being able to treat these threatening pathogens. The most widely recognized serotypes that cause severe foodborne illness are E. coli O157:H7, O26:H11, and O78:H10, and they display increasing antimicrobial resistance rates. Therefore, there is an urgent need for an effective therapy that has dual action to inhibit biofilm formation and decrease bacterial growth. In this study, the synthesized RHL-Fe3O4@PVA@p-CoA/GA biosurfactant NPs have interesting properties, making them excellent candidates for targeted drug delivery by inhibiting bacterial growth and downregulating biofilm-associated IcaABCD and CsgBAC gene loci.
Keywords: antiadhesive property; antimicrobial activity; biofilm formation; drug delivery; iron oxide nanoparticles; rhamnolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

