Effect of Nintedanib on Progression of Systemic Sclerosis-Associated Interstitial Lung Disease Over 100 Weeks: Data From a Randomized Controlled Trial
- PMID: 35852465
- PMCID: PMC9555199
- DOI: 10.1002/acr2.11483
Effect of Nintedanib on Progression of Systemic Sclerosis-Associated Interstitial Lung Disease Over 100 Weeks: Data From a Randomized Controlled Trial
Abstract
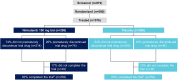
Objective: In the SENSCIS trial, participants with systemic sclerosis-associated interstitial lung disease (SSc-ILD) were randomized to receive nintedanib or placebo until the last participant reached week 52 but for 100 weeks or less. Nintedanib reduced the rate of decline in forced vital capacity (FVC) (ml/year) over 52 weeks by 44% (41 ml [95% confidence interval (95% CI): 2.9-79.0]) versus placebo. We investigated the effect of nintedanib over the whole SENSCIS trial.
Methods: The annual rate of decline in FVC (ml/year) over the whole trial was assessed descriptively using 1) on-treatment data plus off-treatment data from participants who prematurely discontinued treatment (intent-to-treat analysis) and 2) only on-treatment data to assess the effect of nintedanib in participants who remained on treatment.
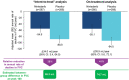
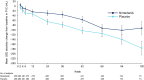
Results: In the intent-to-treat analysis, the adjusted mean (SE) annual rate of decline in FVC over 100 weeks was -54.9 (11.1) and -88.8 (10.9) ml/year in the nintedanib (n = 287) and placebo (n = 288) groups, respectively (difference 34.0 ml/year [95% CI: 3.4-64.5]). In the on-treatment analysis, the adjusted mean (SE) annual rate of decline in FVC over 100 weeks was -55.1 (12.3) and -94.0 (11.7) ml/year in the nintedanib (n = 286) and placebo (n = 288) groups, respectively (difference 38.9 ml/year [95% CI: 5.6-72.1]). The adverse event profile of nintedanib over 100 weeks was consistent with that observed over 52 weeks.
Conclusion: Nintedanib provides a sustained benefit on slowing the progression of SSc-ILD over 100 weeks, with adverse events that are manageable for most patients.
© 2022 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, et al. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. - PubMed
-
- Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir‐Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. - PubMed
-
- Goh NS, Hoyles RK, Denton CP, Hansell DM, Renzoni EA, Maher TM, et al. Short‐term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. - PubMed
-
- Hoffmann‐Vold AM, Fretheim H, Halse AK, Seip M, Bitter H, Wallenius M, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019;200:1258–66. - PubMed

