Activation of the hypoxia response protects mice from amyloid-β accumulation
- PMID: 35852609
- PMCID: PMC9296391
- DOI: 10.1007/s00018-022-04460-6
Activation of the hypoxia response protects mice from amyloid-β accumulation
Abstract
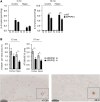
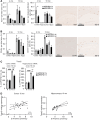
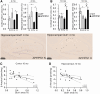
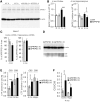
Alzheimer's disease (AD) is the most common cause of dementia with limited treatment options affecting millions of people and the prevalence increasing with the aging population. The current knowledge on the role of the hypoxia/hypoxia-inducible factor (HIF) in the AD pathology is restricted and controversial. We hypothesized based on benefits of the genetic long-term inactivation of HIF prolyl 4-hydroxylase-2 (HIF-P4H-2) on metabolism, vasculature and inflammatory response that prolonged moderate activation of the hypoxia response could hinder AD pathology. We used an aging model to study potential spontaneous accumulation of amyloid-β (Aβ) in HIF-P4H-2-deficient mice and a transgenic APP/PSEN1 mouse model subjected to prolonged sustained environmental hypoxia (15% O2 for 6 weeks) at two different time points of the disease; at age of 4 and 10 months. In both settings, activation of the hypoxia response reduced brain protein aggregate levels and this associated with higher vascularity. In the senescent HIF-P4H-2-deficient mice metabolic reprogramming also contributed to less protein aggregates while in APP/PSEN1 mice lesser Aβ associated additionally with hypoxia-mediated favorable responses to neuroinflammation and amyloid precursor protein processing. In conclusion, continuous, non-full-scale activation of the HIF pathway appears to mediate protection against neurodegeneration via several mechanisms and should be studied as a treatment option for AD.
Keywords: Alzheimer’s disease; HIF; Hypoxia; Inflammation; Metabolism; Vascularity.
© 2022. The Author(s).
Conflict of interest statement
Financial interests: JMy owns equity in FibroGen, Inc., which develops HIF-P4H inhibitors as therapeutics and supports research in JMy laboratory. The other authors have no relevant financial or non-financial interests to disclose.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

