Efficacy of Pseudo-Ceramide-Containing Steroid Lamellar Cream in Patients with Mild to Moderate Atopic Dermatitis: A Randomized, Double-Blind Study
- PMID: 35852694
- PMCID: PMC9357596
- DOI: 10.1007/s13555-022-00766-2
Efficacy of Pseudo-Ceramide-Containing Steroid Lamellar Cream in Patients with Mild to Moderate Atopic Dermatitis: A Randomized, Double-Blind Study
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory skin disorder involving decreased barrier function of the stratum corneum. This decrease, caused by a reduction in ceramide, the primary component of intercellular lipids in the stratum corneum, leads to a disturbance in the lamellar structure.
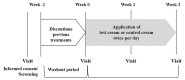
Methods: We developed a formulation (test cream) containing a steroid and synthetic pseudo-ceramide (SLE: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethyl hexadecanamide) that forms a lamellar structure on the skin after its application and drying. The formulation or control cream (a formulation containing a steroid but not pseudo-ceramide that does not form a lamellar structure) was applied twice daily for 2 weeks to the lesional area of 34 participants with mild to moderate AD symptoms.
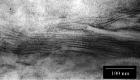
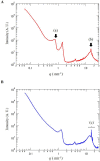
Results: The test cream showed a periodic structure with an interface space of approximately 8.2 nm in transmission electron microscopy and small- and wide-angle X-ray scattering, similar to the lamellar structure in the human stratum corneum. In the double-blind test, the anti-inflammatory effects of the test cream (n = 17) were comparable to those of the control cream (n = 17). In the test cream group, a significant increase in the stratum corneum moisture content (p < 0.01) and significant decrease in transepidermal water loss (p < 0.05) were observed at weeks 1 and 2 after application compared with those before application. No such change was observed in the control group.
Conclusion: The results indicate that, even with a relatively short application period of 2 weeks, the test cream not only suppressed inflammation of the lesional area, but also improved the inherent barrier function of the stratum corneum, suggesting its potential as a treatment option for patients with AD.
Keywords: Atopic dermatitis; Comparative study; Lamella; Pseudo-ceramide; Steroid.
© 2022. The Author(s).
Figures



References
-
- Kikuchi K, Tagami H. Japanese Cosmetic Scientist Task Force for Skin Care of Atopic Dermatitis. Noninvasive biophysical assessments of the efficacy of a moisturizing cosmetic cream base for patients with atopic dermatitis during different seasons. Br J Dermatol. 2008;158:969–978. doi: 10.1111/j.1365-2133.2008.08478.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

