Integrated single-cell transcriptomics and proteomics reveal cellular-specific responses and microenvironment remodeling in aristolochic acid nephropathy
- PMID: 35852860
- PMCID: PMC9462482
- DOI: 10.1172/jci.insight.157360
Integrated single-cell transcriptomics and proteomics reveal cellular-specific responses and microenvironment remodeling in aristolochic acid nephropathy
Abstract
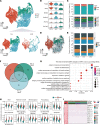
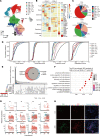
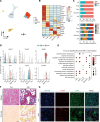
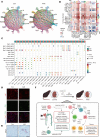
Aristolochic acid nephropathy (AAN) is characterized by acute proximal tubule necrosis and immune cell infiltration, contributing to the global burden of chronic kidney disease and urothelial cancer. Although the proximal tubule has been defined as the primary target of aristolochic acids I (AAI), the mechanistic underpinning of gross renal deterioration caused by AAI has not been explicitly explained, prohibiting effective therapeutic intervention. To this point, we employed integrated single-cell RNA-Seq, bulk RNA-Seq, and mass spectrometry-based proteomics to analyze the mouse kidney after acute AAI exposure. Our results reveal a dramatic reduction of proximal tubule epithelial cells, associated with apoptotic and inflammatory pathways, indicating permanent damage beyond repair. We found the enriched development pathways in other nephron segments, suggesting activation of reparative programs triggered by AAI. The divergent response may be attributed to the segment-specific distribution of organic anion channels along the nephron, including OAT1 and OAT3. Moreover, we observed dramatic activation and recruitment of cytotoxic T and macrophage M1 cells, highlighting inflammation as a principal contributor to permanent renal injury. Ligand-receptor pairing revealed that critical intercellular crosstalk underpins damage-induced activation of immune cells. These results provide potentially novel insight into the AAI-induced kidney injury and point out possible pathways for future therapeutic intervention.
Keywords: Apoptosis; Cell Biology; Mouse models; NF-kappaB; Nephrology.
Figures




References
-
- Wankowicz Z, et al. Nephropathy after use of Chinese herbs: rapidly progressive interstitial renal fibrosis or renal vasculitis? Pol Arch Med Wewn. 1996;96(5):474–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

