The UIP/IPF fibroblastic focus is a collagen biosynthesis factory embedded in a distinct extracellular matrix
- PMID: 35852874
- PMCID: PMC9462507
- DOI: 10.1172/jci.insight.156115
The UIP/IPF fibroblastic focus is a collagen biosynthesis factory embedded in a distinct extracellular matrix
Abstract
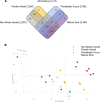
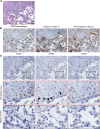
Usual interstitial pneumonia (UIP) is a histological pattern characteristic of idiopathic pulmonary fibrosis (IPF). The UIP pattern is patchy with histologically normal lung adjacent to dense fibrotic tissue. At this interface, fibroblastic foci (FF) are present and are sites where myofibroblasts and extracellular matrix (ECM) accumulate. Utilizing laser capture microdissection-coupled mass spectrometry, we interrogated the FF, adjacent mature scar, and adjacent alveoli in 6 fibrotic (UIP/IPF) specimens plus 6 nonfibrotic alveolar specimens as controls. The data were subjected to qualitative and quantitative analysis and histologically validated. We found that the fibrotic alveoli protein signature is defined by immune deregulation as the strongest category. The fibrotic mature scar classified as end-stage fibrosis whereas the FF contained an overabundance of a distinctive ECM compared with the nonfibrotic control. Furthermore, FF were positive for both TGFB1 and TGFB3, whereas the aberrant basaloid cell lining of FF was predominantly positive for TGFB2. In conclusion, spatial proteomics demonstrated distinct protein compositions in the histologically defined regions of UIP/IPF tissue. These data revealed that FF are the main site of collagen biosynthesis and that the adjacent alveoli are abnormal. This essential information will inform future mechanistic studies on fibrosis progression.
Keywords: Extracellular matrix; Fibrosis; Proteomics; Pulmonology.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

