Endoplasmic reticulum oxidoreductin 1-alpha deficiency and activation of protein translation synergistically impair breast tumour resilience
- PMID: 35853086
- PMCID: PMC9804893
- DOI: 10.1111/bph.15927
Endoplasmic reticulum oxidoreductin 1-alpha deficiency and activation of protein translation synergistically impair breast tumour resilience
Abstract
Background and purpose: Endoplasmic reticulum (ER) stress triggers an adaptive response in tumours which fosters cell survival and resilience to stress. Activation of the ER stress response, through its PERK branch, promotes phosphorylation of the α-subunit of the translation initiation factor eIF2, thereby repressing general protein translation and augmenting the translation of ATF4 with the downstream CHOP transcription factor and the protein disulfide oxidase, ERO1-alpha EXPERIMENTAL APPROACH: Here, we show that ISRIB, a small molecule that inhibits the action of phosphorylated eIF2alpha, activating protein translation, synergistically interacts with the genetic deficiency of protein disulfide oxidase ERO1-alpha, enfeebling breast tumour growth and spread.
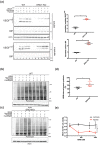
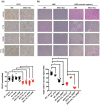
Key results: ISRIB represses the CHOP signal, but does not inhibit ERO1. Mechanistically, ISRIB increases the ER protein load with a marked perturbing effect on ERO1-deficient triple-negative breast cancer cells, which display impaired proteostasis and have adapted to a low client protein load in hypoxia, and ERO1 deficiency impairs VEGF-dependent angiogenesis. ERO1-deficient triple-negative breast cancer xenografts have an augmented ER stress response and its PERK branch. ISRIB acts synergistically with ERO1 deficiency, inhibiting the growth of triple-negative breast cancer xenografts by impairing proliferation and angiogenesis.
Conclusion and implications: These results demonstrate that ISRIB together with ERO1 deficiency synergistically shatter the PERK-dependent adaptive ER stress response, by restarting protein synthesis in the setting of impaired proteostasis, finally promoting tumour cytotoxicity. Our findings suggest two surprising features in breast tumours: ERO1 is not regulated via CHOP under hypoxic conditions, and ISRIB offers a therapeutic option to efficiently inhibit tumour progression in conditions of impaired proteostasis.
Keywords: ERO1 alpha; ISRIB (integrated stress response inhibitor); PERK pathway; UPR (unfolded protein response); breast cancer; endoplasmic reticulum stress.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , & Southan, C. (2021). The concise guide to pharmacology 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(Suppl 1), S264–S312. 10.1111/bph.15541 - DOI - PubMed
-
- Blais, J. D. , Chin, K. T. , Zito, E. , Zhang, Y. , Heldman, N. , Harding, H. P. , Fass, D. , Thorpe, C. , & Ron, D. (2010). A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. The Journal of Biological Chemistry, 285(27), 20993–21003. doi: M110.126599 [pii]. 10.1074/jbc.M110.126599 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

