Synthesis and characterisation of a cancerous liver for presurgical planning and training applications
- PMID: 35853677
- PMCID: PMC9301799
- DOI: 10.1136/bmjgast-2022-000909
Synthesis and characterisation of a cancerous liver for presurgical planning and training applications
Abstract
Objectives: Oncology surgeons use animals and cadavers in training because of a lack of alternatives. The aim of this work was to develop a design methodology to create synthetic liver models familiar to surgeons, and to help plan, teach and rehearse patient-specific cancerous liver resection surgery.
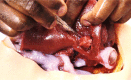
Design: Synthetic gels were selected and processed to recreate accurate anthropomorphic qualities. Organic and synthetic materials were mechanically tested with the same equipment and standards to determine physical properties like hardness, elastic modulus and viscoelasticity. Collected data were compared with published data on the human liver. Patient-specific CT data were segmented and reconstructed and additive manufactured models were made of the liver vasculature, parenchyma and lesion. Using toolmaking and dissolvable scaffolds, models were transformed into tactile duplicates that could mimic liver tissue behaviour.
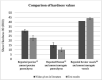
Results: Porcine liver tissue hardness was found to be 23 H00 (±0.1) and synthetic liver was 10 H00 (±2.3), while human parenchyma was reported as 15.06 H00 (±2.64). Average elastic Young's modulus of human liver was reported as 0.012 MPa, and synthetic liver was 0.012 MPa, but warmed porcine parenchyma was 0.28 MPa. The final liver model demonstrated a time-dependant viscoelastic response to cyclic loading.
Conclusion: Synthetic liver was better than porcine liver at recreating the mechanical properties of living human liver. Warmed porcine liver was more brittle, less extensible and stiffer than both human and synthetic tissues. Qualitative surgical assessment of the model by a consultant liver surgeon showed vasculature was explorable and that bimanual palpation, organ delivery, transposition and organ slumping were analogous to human liver behaviour.
Keywords: COLORECTAL METASTASES; HEPATIC SURGERY; HEPATOCELLULAR CARCINOMA; SURGICAL TRAINING.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- Anon. Joint Committee on surgical training (JCST), 2014. CCT guidelines FINAL GS V5. Available: https://www.jcst.org/search/?q=hpb
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
