Association of Rosuvastatin Use with Risk of Hematuria and Proteinuria
- PMID: 35853713
- PMCID: PMC9529194
- DOI: 10.1681/ASN.2022020135
Association of Rosuvastatin Use with Risk of Hematuria and Proteinuria
Abstract
Background: Despite reports of hematuria and proteinuria with rosuvastatin use at the time of its approval by the US Food and Drug Association (FDA), little postmarketing surveillance exists to assess real-world risk. Current labeling suggests dose reduction (maximum daily dose of 10 mg) for patients with severe CKD.
Methods: Using deidentified electronic health record data, we analyzed 152,101 and 795,799 new users of rosuvastatin and atorvastatin, respectively, from 2011 to 2019. We estimated inverse probability of treatment-weighted hazard ratios (HRs) of hematuria, proteinuria, and kidney failure with replacement therapy (KFRT) associated with rosuvastatin. We reported the initial rosuvastatin dose across eGFR categories and evaluated for a dose effect on hematuria and proteinuria.
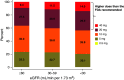
Results: Overall, we identified 2.9% of patients with hematuria and 1.0% with proteinuria during a median follow-up of 3.1 years. Compared with atorvastatin, rosuvastatin was associated with increased risk of hematuria (HR, 1.08; 95% confidence interval [95% CI], 1.04 to 1.11), proteinuria (HR, 1.17; 95% CI, 1.10 to 1.25), and KFRT (HR, 1.15; 95% CI, 1.02 to 1.30). A substantial share (44%) of patients with eGFR <30 ml/min per 1.73 m2 was prescribed high-dose rosuvastatin (20 or 40 mg daily). Risk was higher with higher rosuvastatin dose.
Conclusions: Compared with atorvastatin, rosuvastatin was associated with increased risk of hematuria, proteinuria, and KFRT. Among patients with eGFR <30 ml/min per 1.73 m2, 44% were prescribed a rosuvastatin daily dose exceeding the FDA's recommended 10 mg daily dose. Our findings suggest the need for greater care in prescribing and monitoring rosuvastatin, particularly in patients who receive high doses or who have severe CKD.
Keywords: chronic kidney disease; clinical epidemiology; drug nephrotoxicity; hematuria; proteinuria; rosuvastatin calcium; statins.
Copyright © 2022 by the American Society of Nephrology.
Figures



References
-
- Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. ; STELLAR Study Group : Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 92: 152–160, 2003 - PubMed
-
- The statin wars: Why AstraZeneca must retreat. Lancet 362: 1341, 2003 - PubMed
-
- Cohen JS: Should rosuvastatin be withdrawn from the market? Lancet 364: 1579, 2004 - PubMed
-
- Florentinus SR, Heerdink ER, Klungel OH, de Boer A: Should rosuvastatin be withdrawn from the market? Lancet 364: 1577, 2004 - PubMed
-
- Kastelein JJ: Should rosuvastatin be withdrawn from the market? Lancet 364: 1577–1578, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

