GsαR201C and estrogen reveal different subsets of bone marrow adiponectin expressing osteogenic cells
- PMID: 35853852
- PMCID: PMC9296668
- DOI: 10.1038/s41413-022-00220-1
GsαR201C and estrogen reveal different subsets of bone marrow adiponectin expressing osteogenic cells
Abstract
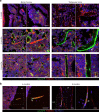
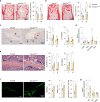
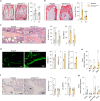
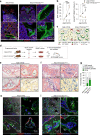
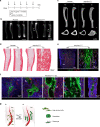
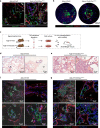
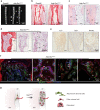
The Gsα/cAMP signaling pathway mediates the effect of a variety of hormones and factors that regulate the homeostasis of the post-natal skeleton. Hence, the dysregulated activity of Gsα due to gain-of-function mutations (R201C/R201H) results in severe architectural and functional derangements of the entire bone/bone marrow organ. While the consequences of gain-of-function mutations of Gsα have been extensively investigated in osteoblasts and in bone marrow osteoprogenitor cells at various differentiation stages, their effect in adipogenically-committed bone marrow stromal cells has remained unaddressed. We generated a mouse model with expression of GsαR201C driven by the Adiponectin (Adq) promoter. Adq-GsαR201C mice developed a complex combination of metaphyseal, diaphyseal and cortical bone changes. In the metaphysis, GsαR201C caused an early phase of bone resorption followed by bone deposition. Metaphyseal bone formation was sustained by cells that were traced by Adq-Cre and eventually resulted in a high trabecular bone mass phenotype. In the diaphysis, GsαR201C, in combination with estrogen, triggered the osteogenic activity of Adq-Cre-targeted perivascular bone marrow stromal cells leading to intramedullary bone formation. Finally, consistent with the previously unnoticed presence of Adq-Cre-marked pericytes in intraosseous blood vessels, GsαR201C caused the development of a lytic phenotype that affected both cortical (increased porosity) and trabecular (tunneling resorption) bone. These results provide the first evidence that the Adq-cell network in the skeleton not only regulates bone resorption but also contributes to bone formation, and that the Gsα/cAMP pathway is a major modulator of both functions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr. Rev. 2001;22:675–705. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

