Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome
- PMID: 35853863
- PMCID: PMC9295877
- DOI: 10.1038/s41467-022-31557-0
Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome
Abstract
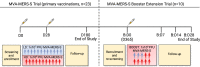
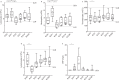
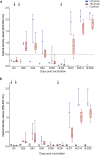
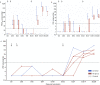
Vaccine development is essential for pandemic preparedness. We previously conducted a Phase 1 clinical trial of the vector vaccine candidate MVA-MERS-S against the Middle East respiratory syndrome coronavirus (MERS-CoV), expressing its full spike glycoprotein (MERS-CoV-S), as a homologous two-dose regimen (Days 0 and 28). Here, we evaluate the safety (primary objective) and immunogenicity (secondary and exploratory objectives: magnitude and characterization of vaccine-induced humoral responses) of a third vaccination with MVA-MERS-S in a subgroup of trial participants one year after primary immunization. MVA-MERS-S booster vaccination is safe and well-tolerated. Both binding and neutralizing anti-MERS-CoV antibody titers increase substantially in all participants and exceed maximum titers observed after primary immunization more than 10-fold. We identify four immunogenic IgG epitopes, located in the receptor-binding domain (RBD, n = 1) and the S2 subunit (n = 3) of MERS-CoV-S. The level of baseline anti-human coronavirus antibody titers does not impact the generation of anti-MERS-CoV antibody responses. Our data support the rationale of a booster vaccination with MVA-MERS-S and encourage further investigation in larger trials. Trial registration: Clinicaltrials.gov NCT03615911.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. Prioritizing diseases for research and development in emergency contexts, https://www.who.int/activities/prioritizing-diseases-for-research-and-de... (2021).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

