α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice
- PMID: 35853942
- PMCID: PMC9296447
- DOI: 10.1038/s41467-022-31787-2
α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice
Abstract
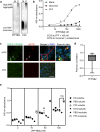
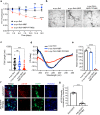
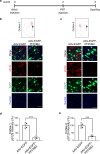
Pathogenic α-synuclein (α-syn) is a prion-like protein that drives the pathogenesis of Lewy Body Dementia (LBD) and Parkinson's Disease (PD). To target pathogenic α-syn preformed fibrils (PFF), here we designed extracellular disulfide bond-free synthetic nanobody libraries in yeast. Following selection, we identified a nanobody, PFFNB2, that can specifically recognize α-syn PFF over α-syn monomers. PFFNB2 cannot inhibit the aggregation of α-syn monomer, but can significantly dissociate α-syn fibrils. Furthermore, adeno-associated virus (AAV)-encoding EGFP fused to PFFNB2 (AAV-EGFP-PFFNB2) can inhibit PFF-induced α-syn serine 129 phosphorylation (pS129) in mouse primary cortical neurons, and prevent α-syn pathology spreading to the cortex in the transgenic mice expressing human wild type (WT) α-syn by intrastriatal-PFF injection. The pS129 immunoreactivity is negatively correlated with the expression of AAV-EGFP-PFFNB2. In conclusion, PFFNB2 holds a promise for mechanistic exploration and therapeutic development in α-syn-related pathogenesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: patent application filed by W.W., X.M., Y.B., Y.L., and R.K. with title ‘Compositions and methods for treating alpha-synucleinopathies.’ U.S. Provisional Patent Application No. 63/222,141, filed Jul 15, 2021. Applicants: The Regents of the University of Michigan and The Johns Hopkins University. Patent pending. Some aspects of this paper is included in the patent such as disulfide-bond free PFFNB, AAV delivery of PFFNB2 to treat Parkinson’s Disease. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

