Mechanisms and consequences of endothelial cell senescence
- PMID: 35853997
- PMCID: PMC10026597
- DOI: 10.1038/s41569-022-00739-0
Mechanisms and consequences of endothelial cell senescence
Abstract
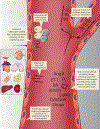
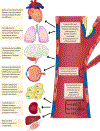
Endothelial cells are located at the crucial interface between circulating blood and semi-solid tissues and have many important roles in maintaining systemic physiological function. The vascular endothelium is particularly susceptible to pathogenic stimuli that activate tumour suppressor pathways leading to cellular senescence. We now understand that senescent endothelial cells are highly active, secretory and pro-inflammatory, and have an aberrant morphological phenotype. Moreover, endothelial senescence has been identified as an important contributor to various cardiovascular and metabolic diseases. In this Review, we discuss the consequences of endothelial cell exposure to damaging stimuli (haemodynamic forces and circulating and endothelial-derived factors) and the cellular and molecular mechanisms that induce endothelial cell senescence. We also discuss how endothelial cell senescence causes arterial dysfunction and contributes to clinical cardiovascular diseases and metabolic disorders. Finally, we summarize the latest evidence on the effect of eliminating senescent endothelial cells (senolysis) and identify important remaining questions to be addressed in future studies.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
A.J.D. is a scientific advisor and stockholder in Recursion Pharmaceuticals. None of the work done with Recursion is outlined or discussed in this Review. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

