Vitamin C and folate status in hereditary fructose intolerance
- PMID: 35854131
- PMCID: PMC9708598
- DOI: 10.1038/s41430-022-01178-3
Vitamin C and folate status in hereditary fructose intolerance
Erratum in
-
Correction: Vitamin C and folate status in hereditary fructose intolerance.Eur J Clin Nutr. 2023 Nov;77(11):1102-1103. doi: 10.1038/s41430-023-01334-3. Eur J Clin Nutr. 2023. PMID: 37673953 Free PMC article. No abstract available.
Abstract
Background: Hereditary fructose intolerance (HFI) is a rare inborn error of fructose metabolism caused by the deficiency of aldolase B. Since treatment consists of a fructose-, sucrose- and sorbitol-restrictive diet for life, patients are at risk of presenting vitamin deficiencies. Although there is no published data on the status of these vitamins in HFI patients, supplementation with vitamin C and folic acid is common. Therefore, the aim of this study was to assess vitamin C and folate status and supplementation practices in a nationwide cohort of HFI patients.
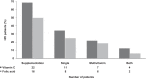
Methods: Vitamin C and folic acid dietary intake, supplementation and circulating levels were assessed in 32 HFI patients and 32 age- and sex-matched healthy controls.
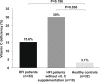
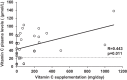
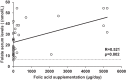
Results: Most of the HFI participants presented vitamin C (96.7%) and folate (90%) dietary intake below the recommended population reference intake. Up to 69% received vitamin C and 50% folic acid supplementation. Among HFI patients, 15.6% presented vitamin C and 3.1% folate deficiency. The amount of vitamin C supplementation and plasma levels correlated positively (R = 0.443; p = 0.011). Interestingly, a higher percentage of non-supplemented HFI patients were vitamin C deficient when compared to supplemented HFI patients (30% vs. 9.1%; p = 0.01) and to healthy controls (30% vs. 3.1%; p < 0.001).
Conclusions: Our results provide evidence for the first time supporting vitamin C supplementation in HFI. There is great heterogeneity in vitamin supplementation practices and, despite follow-up at specialised centres, vitamin C deficiency is common. Further research is warranted to establish optimal doses of vitamin C and the need for folic acid supplementation in HFI.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Morris RC. An experimental renal acidification defect in patients with hereditary fructose intolerance: II. Its distinction from classic renal tubular acidosis; its resemblance to the renal acidification defect associated with the fanconi syndrome of children with cystinosis. J Clin Investig. 1968;47:1648–63. doi: 10.1172/JCI105856. - DOI - PMC - PubMed
-
- Baerlocher K, Gitzelmann R, Steinmann B, Gitzelmann-Cumarasamy N. Hereditary fructose intolerance in early childhood: a major diagnostic challenge. Survey of 20 symptomatic cases. Helvetica Paediatrica Acta. 1978;33:465–87. - PubMed

