Clinical profiles of treated and untreated adults with hypophosphatasia in the Global HPP Registry
- PMID: 35854311
- PMCID: PMC9295501
- DOI: 10.1186/s13023-022-02393-8
Clinical profiles of treated and untreated adults with hypophosphatasia in the Global HPP Registry
Abstract
Background: The clinical signs and symptoms of hypophosphatasia (HPP) can manifest during any stage of life. The age at which a patient's symptoms are reported can impact access to targeted treatment with enzyme replacement therapy (asfotase alfa), as this treatment is indicated for patients with pediatric-onset HPP in most countries. As such, many patients reported to have adult-onset HPP typically do not receive treatment. Comparison of the disease in treated and untreated adult patients is confounded by the approved indication. To avoid this confounding factor, a comparison between baseline disease manifestations prominent among treated versus untreated adult patients was limited to those with pediatric-onset HPP using data collected from the Global HPP Registry. The hypothesis was that treated adults will have a greater disease burden at baseline than untreated adults. The analysis of disease manifestations in adults with adult-onset HPP was conducted separately.
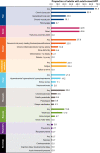
Results: A total of 398 adults with HPP were included; 213 with pediatric-onset (114 treated, 99 untreated) and 141 with adult-onset HPP (2 treated and 139 untreated). The treated, pediatric-onset patients were more likely to have a history of pain (prevalence ratio [PR]: 1.3, 95% confidence interval [CI] 1.1, 1.4), skeletal (PR: 1.3, 95% CI 1.1, 1.6), constitutional/metabolic (PR: 1.7, 95% CI 1.3, 2.0), muscular (PR: 1.8, 95% CI 1.4, 2.1) and neurological (PR: 1.7, 95% CI 1.1, 2.3) manifestations of HPP, and also had poorer measures for health-related quality of life, pain, and disability compared with untreated pediatric-onset patients. In patients with adult-onset HPP, the most frequent signs and symptoms were chronic bone pain (52.5%), dental manifestations (42.6%), fatigue (23.4%), recurrent fractures or pseudofractures (22.0%), and generalized body pain (22.0%).
Conclusions: Along with the more classical skeletal signs and symptoms, pain, muscular, and constitutional/metabolic manifestations are common in adults with HPP, regardless of age of disease onset, highlighting a full spectrum of HPP manifestations.
Keywords: Burden of disease; Enzyme replacement therapy; HRQoL; Hypophosphatasia.
© 2022. The Author(s).
Conflict of interest statement
Anna Petryk and Shona Fang are employees of, and may own stock/options in, Alexion, AstraZeneca Rare Disease. Priya S Kishnani, Gabriel Ángel Martos-Moreno, Kathryn M Dahir, Agnès Linglart, Cheryl Rockman-Greenberg, Keiichi Ozono, Lothar Seefried, and Wolfgang Högler are consultants for, and have received research funding and honoraria from, Alexion, AstraZeneca Rare Disease.
Figures



References
-
- The ALPL gene variant database. https://alplmutationdatabase.jku.at/ Accessed February 10, 2022.
-
- Rasmussen K. Phosphorylethanolamine and hypophosphatasia. Dan Med Bull. 1968;15(Suppl 2):1–112. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

