Systemic or Vaginal Hormone Therapy After Early Breast Cancer: A Danish Observational Cohort Study
- PMID: 35854422
- PMCID: PMC9552278
- DOI: 10.1093/jnci/djac112
Systemic or Vaginal Hormone Therapy After Early Breast Cancer: A Danish Observational Cohort Study
Abstract
Background: Women treated for breast cancer (BC) often suffer genitourinary syndrome of menopause. These symptoms may be alleviated by vaginal estrogen therapy (VET) or menopausal hormone therapy (MHT). However, there are concerns of risks of recurrence of BC and death following treatment.
Methods: Our study included longitudinal data from a national cohort of postmenopausal women, diagnosed 1997-2004 with early-stage invasive estrogen receptor-positive nonmetastatic BC, who received no treatment or 5 years of adjuvant endocrine therapy. We ascertained prescription data on hormone therapy, VET or MHT, from a national prescription registry. We evaluated mortality and risk of recurrence associated with use of VET and MHT vs non-use using multivariable models adjusted for potential confounders.
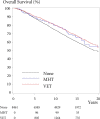
Results: Among 8461 women who had not received VET or MHT before BC diagnosis, 1957 and 133 used VET and MHT, respectively, after diagnosis. Median follow-up was 9.8 years for recurrence and 15.2 years for mortality. The adjusted relative risk of recurrence was 1.08 (95% confidence interval [CI] = 0.89 to 1.32) for VET (1.39 [95% CI = 1.04 to 1.85 in the subgroup receiving adjuvant aromatase inhibitors]) and 1.05 (95% CI = 0.62 to 1.78) for MHT. The adjusted hazard ratios for overall mortality were 0.78 (95% CI = 0.71 to 0.87) and 0.94 (95% CI = 0.70 to 1.26) for VET and MHT, respectively.
Conclusions: In postmenopausal women treated for early-stage estrogen receptor-positive BC, neither VET nor MHT was associated with increased risk of recurrence or mortality. A subgroup analysis revealed an increased risk of recurrence, but not mortality, in patients receiving VET with adjuvant aromatase inhibitors.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Comment in
-
Vaginal Estrogen Therapy for the Genitourinary Symptoms of Menopause: Caution or Reassurance?J Natl Cancer Inst. 2022 Oct 6;114(10):1315-1316. doi: 10.1093/jnci/djac113. J Natl Cancer Inst. 2022. PMID: 35854417 Free PMC article. No abstract available.
-
RE: Systemic or vaginal hormone therapy after early breast cancer: a Danish observational cohort study.J Natl Cancer Inst. 2023 Feb 8;115(2):220-221. doi: 10.1093/jnci/djac211. J Natl Cancer Inst. 2023. PMID: 36409007 Free PMC article. No abstract available.
-
RE: Systemic or vaginal hormone therapy after early breast cancer: a Danish observational cohort study.J Natl Cancer Inst. 2023 Feb 8;115(2):222-223. doi: 10.1093/jnci/djac213. J Natl Cancer Inst. 2023. PMID: 36409032 Free PMC article. No abstract available.
-
RE: Systemic or vaginal hormone therapy after early breast cancer: a Danish Observational Cohort Study.J Natl Cancer Inst. 2023 Aug 8;115(8):998-999. doi: 10.1093/jnci/djad089. J Natl Cancer Inst. 2023. PMID: 37216905 Free PMC article. No abstract available.
References
-
- Frechette D, Paquet L, Verma S, et al. The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: encouraging results from a prospective study. Breast Cancer Res Treat. 2013;141(1):111-117. - PubMed
-
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063-1068. - PubMed
-
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888-902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

