Three-dimensional-printed collagen/chitosan/secretome derived from HUCMSCs scaffolds for efficient neural network reconstruction in canines with traumatic brain injury
- PMID: 35855109
- PMCID: PMC9290528
- DOI: 10.1093/rb/rbac043
Three-dimensional-printed collagen/chitosan/secretome derived from HUCMSCs scaffolds for efficient neural network reconstruction in canines with traumatic brain injury
Abstract
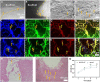
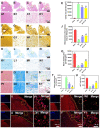
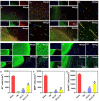
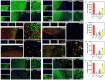
The secretome secreted by stem cells and bioactive material has emerged as a promising therapeutic choice for traumatic brain injury (TBI). We aimed to determine the effect of 3D-printed collagen/chitosan/secretome derived from human umbilical cord blood mesenchymal stem cells scaffolds (3D-CC-ST) on the injured tissue regeneration process. 3D-CC-ST was performed using 3D printing technology at a low temperature (-20°C), and the physical properties and degeneration rate were measured. The utilization of low temperature contributed to a higher cytocompatibility of fabricating porous 3D architectures that provide a homogeneous distribution of cells. Immediately after the establishment of the canine TBI model, 3D-CC-ST and 3D-CC (3D-printed collagen/chitosan scaffolds) were implanted into the cavity of TBI. Following implantation of scaffolds, neurological examination and motor evoked potential detection were performed to analyze locomotor function recovery. Histological and immunofluorescence staining were performed to evaluate neuro-regeneration. The group treated with 3D-CC-ST had good performance of behavior functions. Implanting 3D-CC-ST significantly reduced the cavity area, facilitated the regeneration of nerve fibers and vessel reconstruction, and promoted endogenous neuronal differentiation and synapse formation after TBI. The implantation of 3D-CC-ST also markedly reduced cell apoptosis and regulated the level of systemic inflammatory factors after TBI.
Keywords: canines; chitosan; collagen; low temperature extrusion 3D printing; secretome; traumatic brain injury.
© The Author(s) 2022. Published by Oxford University Press.
Figures



Similar articles
-
3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats.Stem Cell Res Ther. 2022 Dec 19;13(1):525. doi: 10.1186/s13287-022-03208-0. Stem Cell Res Ther. 2022. PMID: 36536463 Free PMC article.
-
3D-printed collagen/silk fibroin/secretome derived from bFGF-pretreated HUCMSCs scaffolds enhanced therapeutic ability in canines traumatic brain injury model.Front Bioeng Biotechnol. 2022 Aug 24;10:995099. doi: 10.3389/fbioe.2022.995099. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36091465 Free PMC article.
-
Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury.Regen Biomater. 2022 Oct 26;10:rbac085. doi: 10.1093/rb/rbac085. eCollection 2023. Regen Biomater. 2022. PMID: 36683754 Free PMC article.
-
Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines.Front Bioeng Biotechnol. 2022 Sep 30;10:1025138. doi: 10.3389/fbioe.2022.1025138. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246376 Free PMC article.
-
Recent Advances in the Design of Three-Dimensional and Bioprinted Scaffolds for Full-Thickness Wound Healing.Tissue Eng Part B Rev. 2022 Feb;28(1):160-181. doi: 10.1089/ten.TEB.2020.0339. Epub 2021 Feb 22. Tissue Eng Part B Rev. 2022. PMID: 33446047 Review.
Cited by
-
Biomaterial strategies for regulating the neuroinflammatory response.Mater Adv. 2024 Apr 10;5(10):4025-4054. doi: 10.1039/d3ma00736g. eCollection 2024 May 20. Mater Adv. 2024. PMID: 38774837 Free PMC article. Review.
-
Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury.Neural Regen Res. 2023 Sep;18(9):1990-1998. doi: 10.4103/1673-5374.366497. Neural Regen Res. 2023. PMID: 36926724 Free PMC article.
-
Breaking barriers in trauma research: A narrative review of opportunities to leverage veterinary trauma for accelerated translation to clinical solutions for pets and people.J Clin Transl Sci. 2024 Apr 5;8(1):e74. doi: 10.1017/cts.2024.513. eCollection 2024. J Clin Transl Sci. 2024. PMID: 38715566 Free PMC article. Review.
-
The paradigm of stem cell secretome in tissue repair and regeneration: Present and future perspectives.Wound Repair Regen. 2025 Jan-Feb;33(1):e13251. doi: 10.1111/wrr.13251. Wound Repair Regen. 2025. PMID: 39780313 Free PMC article. Review.
-
Collagen protein-chitosan nerve conduits with neuroepithelial stem cells promote peripheral nerve regeneration.Sci Rep. 2024 Sep 5;14(1):20748. doi: 10.1038/s41598-024-71435-x. Sci Rep. 2024. PMID: 39237597 Free PMC article.
References
-
- van Vliet EA, Ndode-Ekane XE, Lehto LJ, Gorter JA, Andrade P, Aronica E, Gröhn O, Pitkänen A.. Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol Dis 2020;145:105080. - PubMed
-
- Qian F, Han Y, Han Z, Zhang D, Zhang L, Zhao G, Li S, Jin G, Yu R, Liu H.. In situ implantable, post-trauma microenvironment-responsive, ROS depletion hydrogels for the treatment of traumatic brain injury. Biomaterials 2021;270:120675. - PubMed
-
- Sultan MT, Choi BY, Ajiteru O, Hong DK, Lee SM, Kim HJ, Ryu JS, Lee JS, Hong H, Lee YJ, Lee H, Suh YJ, Lee OJ, Kim SH, Suh SW, Park CH.. Reinforced-hydrogel encapsulated hMSCs towards brain injury treatment by trans-septal approach. Biomaterials 2021;266:120413. - PubMed
-
- Maas A, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje E, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Söderberg J, Stanworth SJ, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang K, Williams WH, Wilson L, Yaffe K; InTBIR Participants and Investigators. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. - PubMed
LinkOut - more resources
Full Text Sources

