Brain Mitochondrial Dysfunction: A Possible Mechanism Links Early Life Anxiety to Alzheimer's Disease in Later Life
- PMID: 35855329
- PMCID: PMC9286915
- DOI: 10.14336/AD.2022.0221
Brain Mitochondrial Dysfunction: A Possible Mechanism Links Early Life Anxiety to Alzheimer's Disease in Later Life
Abstract
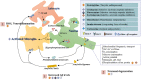
Alzheimer's disease (AD) is usually manifested in patients with dementia, accompanied by anxiety and other mental symptoms. Emerging evidence from humans indicates that people who suffer from anxiety in their early life are more likely to develop AD in later life. Mitochondria, the prominent organelles of energy production in the brain, have crucial physiological significance for the brain, requiring considerable energy to maintain its normal physiological activities. Net reactive oxygen species (ROS) was produced by mitochondrial impairment, in which oxidative stress is also included, and the production of ROS is mostly more than that of removal. In this paper, we propose that as a critical process in brain pathology, mitochondrial dysfunction caused by anxiety triggering oxidative stress might be a possible mechanism that links early life anxiety to AD in later life. Several pivotal physiological roles of mitochondria are reviewed, including functions regulating glucose homeostasis, which may disrupt in oxidative stress. Increased levels of oxidative stress are constantly shown in anxiety disorder patients, and antioxidant drugs have promise in treating anxiety. In the early stages of AD, mitochondrial dysfunction is concentrated around senile plaques, a landmark lesion composed of aggregated Aβ and Tau protein. In turn, the accumulated Aβ and Tau disrupts mitochondrial activity, and the tricky physiological processes of mitochondria might be significant to the course of AD. In the end, we conclude that mitochondria might present as one of the novel therapeutic targets to block oxidative stress in patients with anxiety disorders to prevent AD in the early stage.
Keywords: Alzheimer’s disease; anxiety; early life; mitochondrial dysfunction; oxidative stress.
copyright: © 2022 Wang et al.
Conflict of interest statement
Competing interests All authors report no biomedical financial interests or potential conflicts of interest.
Figures
Similar articles
-
Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer's disease.J Neurol Sci. 2021 Feb 15;421:117253. doi: 10.1016/j.jns.2020.117253. Epub 2020 Dec 5. J Neurol Sci. 2021. PMID: 33476985 Review.
-
Oxidative Stress, Synaptic Dysfunction, and Alzheimer's Disease.J Alzheimers Dis. 2017;57(4):1105-1121. doi: 10.3233/JAD-161088. J Alzheimers Dis. 2017. PMID: 28059794 Free PMC article. Review.
-
Brain capillaries in Alzheimer's disease.Hell J Nucl Med. 2015 Sep-Dec;18 Suppl 1:152. Hell J Nucl Med. 2015. PMID: 26665235
-
Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-β and tau pathology in iPSC-derived neurons from sporadic Alzheimer's disease patients.Stem Cell Res. 2018 Mar;27:121-130. doi: 10.1016/j.scr.2018.01.019. Epub 2018 Jan 28. Stem Cell Res. 2018. PMID: 29414602
-
Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14670-5. doi: 10.1073/pnas.0903563106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667196 Free PMC article.
Cited by
-
The Role of Mitochondrial Dysfunction in Alzheimer's: Molecular Defects and Mitophagy-Enhancing Approaches.Life (Basel). 2023 Apr 8;13(4):970. doi: 10.3390/life13040970. Life (Basel). 2023. PMID: 37109499 Free PMC article. Review.
-
Investigating shared risk variants and genetic etiology between Alzheimer's disease and three stress-related psychiatric disorders: a large-scale genome-wide cross-trait analysis.Front Aging. 2025 Feb 5;6:1488528. doi: 10.3389/fragi.2025.1488528. eCollection 2025. Front Aging. 2025. PMID: 39975850 Free PMC article.
-
Natural antioxidants that act against Alzheimer's disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action.Front Endocrinol (Lausanne). 2023 Jul 3;14:1217730. doi: 10.3389/fendo.2023.1217730. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37465125 Free PMC article. Review.
-
Mitochondrial Transfer in the Neurovascular Unit, Not Only for Energy Rescue: A Systematic Review.Aging Dis. 2024 Jul 16;16(4):2008-2035. doi: 10.14336/AD.2024.0461. Aging Dis. 2024. PMID: 39133904 Free PMC article.
-
Succinylation modification: a potential therapeutic target in stroke.Neural Regen Res. 2024 Apr;19(4):781-787. doi: 10.4103/1673-5374.382229. Neural Regen Res. 2024. PMID: 37843212 Free PMC article. Review.
References
-
- Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU (2016). Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev, 96:1169-1209. - PubMed
-
- Van den Bergh BR (2011). Developmental programming of early brain and behaviour development and mental health: A conceptual framework. Dev Med Child Neurol, 53Suppl 4:19-23. - PubMed
LinkOut - more resources
Full Text Sources
