Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer's Disease
- PMID: 35855330
- PMCID: PMC9286902
- DOI: 10.14336/AD.2021.1215
Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer's Disease
Abstract
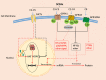
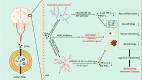
Short-chain fatty acids (SCFAs) are important metabolites derived from the gut microbiota through fermentation of dietary fiber. SCFAs participate a number of physiological and pathological processes in the human body, such as host metabolism, immune regulation, appetite regulation. Recent studies on gut-brain interaction have shown that SCFAs are important mediators of gut-brain interactions and are involved in the occurrence and development of many neurodegenerative diseases, including Alzheimer's disease. This review summarizes the current research on the potential roles and mechanisms of SCFAs in AD. First, we introduce the metabolic distribution, specific receptors and signaling pathways of SCFAs in human body. The concentration levels of SCFAs in AD patient/animal models are then summarized. In addition, we illustrate the effects and mechanisms of SCFAs on the cognitive level, pathological features (Aβ and tau) and neuroinflammation in AD. Finally, we analyze the translational value of SCFAs as potential therapeutic targets for the treatment of AD.
Keywords: Alzheimer's disease; gut microbiota; short-chain fatty acids.
copyright: © 2022 Qian et al.
Conflict of interest statement
Competing interests The authors declare no conflicts of interest.
Figures
Similar articles
-
Gut microbiota-derived short chain fatty acids act as mediators of the gut-brain axis targeting age-related neurodegenerative disorders: a narrative review.Crit Rev Food Sci Nutr. 2025;65(2):265-286. doi: 10.1080/10408398.2023.2272769. Epub 2023 Oct 27. Crit Rev Food Sci Nutr. 2025. PMID: 37897083 Review.
-
Short-chain fatty acids: Important components of the gut-brain axis against AD.Biomed Pharmacother. 2024 Jun;175:116601. doi: 10.1016/j.biopha.2024.116601. Epub 2024 May 14. Biomed Pharmacother. 2024. PMID: 38749177 Review.
-
Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer's Disease.J Neurosci. 2023 Sep 13;43(37):6460-6475. doi: 10.1523/JNEUROSCI.0724-23.2023. Epub 2023 Aug 18. J Neurosci. 2023. PMID: 37596052 Free PMC article.
-
Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article.
-
Therapeutic potential of short-chain fatty acid production by gut microbiota in neurodegenerative disorders.Nutr Res. 2022 Oct;106:72-84. doi: 10.1016/j.nutres.2022.07.007. Epub 2022 Aug 12. Nutr Res. 2022. PMID: 36152586 Review.
Cited by
-
Relationship Between Short-chain Fatty Acids and Parkinson's Disease: A Review from Pathology to Clinic.Neurosci Bull. 2024 Apr;40(4):500-516. doi: 10.1007/s12264-023-01123-9. Epub 2023 Sep 27. Neurosci Bull. 2024. PMID: 37755674 Free PMC article. Review.
-
Gut microbiota-host lipid crosstalk in Alzheimer's disease: implications for disease progression and therapeutics.Mol Neurodegener. 2024 Apr 16;19(1):35. doi: 10.1186/s13024-024-00720-0. Mol Neurodegener. 2024. PMID: 38627829 Free PMC article. Review.
-
The gut-brain-metabolic axis: exploring the role of microbiota in insulin resistance and cognitive function.Front Microbiol. 2024 Nov 26;15:1463958. doi: 10.3389/fmicb.2024.1463958. eCollection 2024. Front Microbiol. 2024. PMID: 39659426 Free PMC article. Review.
-
Prognostic Value of a Multivariate Gut Microbiome Model for Progression from Normal Cognition to Mild Cognitive Impairment Within 4 Years.Int J Mol Sci. 2025 May 15;26(10):4735. doi: 10.3390/ijms26104735. Int J Mol Sci. 2025. PMID: 40429881 Free PMC article.
-
Common Signaling Pathways Involved in Alzheimer's Disease and Stroke: Two Faces of the Same Coin.J Alzheimers Dis Rep. 2023 May 12;7(1):381-398. doi: 10.3233/ADR-220108. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 37220617 Free PMC article. Review.
References
-
- Patterson C (2018). World Alzheimer Report 2018 The state of the art of dementia research: New frontiers.
-
- Kvavilashvili L, Niedzwienska A, Gilbert SJ, Markostamou I (2020). Deficits in Spontaneous Cognition as an Early Marker of Alzheimer's Disease. Trends Cogn Sci, 24:285-301. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
