Bioanalytical assay for the quantification of the tyrosine kinase inhibitor EAI045 and its major metabolite PIA in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry
- PMID: 35855648
- PMCID: PMC9786734
- DOI: 10.1002/bmc.5457
Bioanalytical assay for the quantification of the tyrosine kinase inhibitor EAI045 and its major metabolite PIA in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry
Abstract
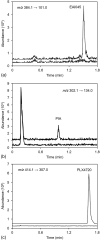
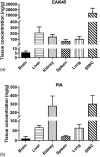
EAI045 is a tyrosine kinase inhibitor (TKI) that targets the mutant epidermal growth factor receptor (EGFR). It was developed to control resistance to available EGFR TKIs. In this study, a major metabolite of EAI045, (5-fluoro-2-hydroxyphenyl)(1-oxo-1,3-dihydro-2H-isoindol-2-yl)acetic acid (PIA), was discovered as a hydrolysis product of the parent drug. A validated assay for both analytes in mouse plasma and tissue homogenates from brain, kidney, liver, lung, spleen, and small intestine with content was set up using LC-MS/MS. Samples were prepared by protein precipitation with acetonitrile and with PLX4720 as internal standard. Separation was performed on a bridged ethylene hybrid C18 column by gradient elution with 0.1% v/v formic acid and methanol. Using positive electrospray, detection was performed in selected reaction monitoring mode. A linear calibration range of 2-2,000 ng/ml was used and validated for both analytes. Precision values ranged between 2.0 and 7.5% for EAI045 and between 2.2 and 12.1% for the metabolite, and accuracy values were between 91.1 and 107.6% for EAI045 and between 87.6 and 100.6% for the metabolite. Both analytes were sufficiently stable under the relevant analytical conditions. Finally, the assay was applied to analyze mouse plasma and tissue levels in a pharmacokinetic study in FVB/NRj wild-type female mice treated with oral EAI045.
Keywords: EAI045; EGFR-TKI; LC-MS/MS; metabolite; mouse matrices.
© 2022 The Authors. Biomedical Chromatography published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Quantification of FGFR4 inhibitor BLU-554 in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Mar 15;1110-1111:116-123. doi: 10.1016/j.jchromb.2019.02.017. Epub 2019 Feb 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2019. PMID: 30802754
-
Quantitative bioanalytical assay for the tropomyosin receptor kinase inhibitor larotrectinib in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Dec 1;1102-1103:167-172. doi: 10.1016/j.jchromb.2018.10.023. Epub 2018 Oct 30. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 30396050
-
An UPLC-MS/MS method for the determination of EAI045 in plasma and tissues and its application to pharmacokinetic and distribution studies in rats.Pharmazie. 2018 Nov 1;73(11):630-634. doi: 10.1691/ph.2018.8123. Pharmazie. 2018. PMID: 30396380
-
Quantitative bioanalytical assay for the selective RET inhibitors selpercatinib and pralsetinib in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2020 Jun 15;1147:122131. doi: 10.1016/j.jchromb.2020.122131. Epub 2020 May 1. J Chromatogr B Analyt Technol Biomed Life Sci. 2020. PMID: 32416592
-
Development and validation of a bioanalytical method for the quantification of the CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib in human and mouse matrices using liquid chromatography-tandem mass spectrometry.Anal Bioanal Chem. 2019 Aug;411(20):5331-5345. doi: 10.1007/s00216-019-01932-w. Epub 2019 Jun 17. Anal Bioanal Chem. 2019. PMID: 31209549 Free PMC article.
Cited by
-
P-Glycoprotein (MDR1/ABCB1) Restricts Brain Accumulation of the Novel EGFR Inhibitor EAI045 and Oral Elacridar Coadministration Enhances Its Brain Accumulation and Oral Exposure.Pharmaceuticals (Basel). 2022 Sep 8;15(9):1124. doi: 10.3390/ph15091124. Pharmaceuticals (Basel). 2022. PMID: 36145346 Free PMC article.
References
-
- Brandon, E. F. A. , Van Ooijen, R. D. , Sparidans, R. W. , López Lázaro, L. , Heck, A. J. R. , Beijnen, J. H. , & Schellens, J. H. M. (2005). Structure elucidation of aplidine metabolites formed in vitro by human liver microsomes using triple quadrupole mass spectrometry. Journal of Mass Spectrometry, 40(6), 821–831. 10.1002/jms.863 - DOI - PubMed
-
- Dong, R. F. , Zhu, M. L. , Liu, M. M. , Xu, Y. T. , Yuan, L. L. , Bian, J. , Xia, Y. Z. , & Kong, L. Y. (2021). EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. In Pharmacological research (Vol. 167). Academic Press. 10.1016/j.phrs.2021.105583 - DOI - PubMed
-
- European Medicines Agency . (2011). 2** Committee for Medicinal Products for Human Use (CHMP) Guideline on bioanalytical method validation. https://www.ema.europa.eu/contact
-
- FDA , & CDER . (2018). Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidanc...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

