Microbial Inactivation: Gaseous or Aqueous Ozonation?
- PMID: 35855724
- PMCID: PMC9284554
- DOI: 10.1021/acs.iecr.2c01551
Microbial Inactivation: Gaseous or Aqueous Ozonation?
Abstract
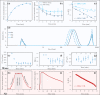
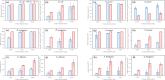
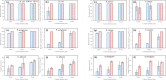
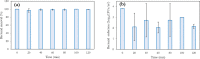
For decades, ozone has been known to have antimicrobial properties when dissolved or generated in water and when utilized in its gaseous form on different substrates. This property (the ability to be used in air and water) makes it versatile and applicable to different industries. Although the medium of ozonation depends on the specific process requirements, some industries have the inherent flexibility of medium selection. Thus, it is important to evaluate the antimicrobial efficacy in both media at similar concentrations, an endeavor hardly reported in the literature. This study provides insights into ozone's efficacy in air and water using two Gram-negative bacteria (Escherichia coli NTCC1290 and Pseudomonas aeruginosa NCTC10332), two Gram-positive bacteria (Staphylococcus aureus ATCC25923 and Streptococcus mutans), and two fungi (Candida albicans and Aspergillus fumigatus). For gaseous ozonation, we utilized a custom-made ozone chamber (equipped with ultraviolet lamps), whereas an electrolysis oxygen radical generator was applied for ozone generation in water. During gaseous ozonation, the contaminated substrates (fabric swatches inoculated with bacterial and fungal suspensions) were suspended in the chamber, whereas the swatches were immersed in ozonated water for aqueous ozone treatment. The stability of ozone nanobubbles and their resulting impact on the aqueous disinfection efficiency were studied via dynamic light scattering measurements. It was observed that ozone is more effective in air than in water on all tested organisms except Staphylococcus aureus. The presented findings allow for the adjustment of the treatment conditions (exposure time and concentration) for optimal decontamination, particularly when a certain medium is preferred for ozonation.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Spencer W.Introduction to Decontamination and Sterilisation. In Manual of Perioperative Care. An Essential Guide; Wiley, 2013.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
