Practical Management for Use of Eculizumab in the Treatment of Severe, Refractory, Non-Thymomatous, AChR + Generalized Myasthenia Gravis: A Systematic Review
- PMID: 35855752
- PMCID: PMC9288180
- DOI: 10.2147/TCRM.S266031
Practical Management for Use of Eculizumab in the Treatment of Severe, Refractory, Non-Thymomatous, AChR + Generalized Myasthenia Gravis: A Systematic Review
Erratum in
-
Erratum: Practical Management for Use of Eculizumab in the Treatment of Severe, Refractory, Non-Thymomatous, AChR + Generalized Myasthenia Gravis: A Systematic Review [Erratum].Ther Clin Risk Manag. 2022 Aug 3;18:773-774. doi: 10.2147/TCRM.S384532. eCollection 2022. Ther Clin Risk Manag. 2022. PMID: 35958346 Free PMC article.
Abstract
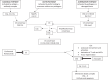
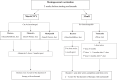
Myasthenia gravis (MG) is a rare autoimmune disorder caused by specific autoantibodies at the neuromuscular junction. MG is classified by the antigen specificity of these antibodies. Acetylcholine receptor (AChR) antibodies are the most common type (74-88%), followed by anti-muscle specific kinase (MuSK) and other antibodies. While all these antibodies lead to neuromuscular transmission failure, the immuno-pathogenic mechanisms are distinct. Complement activation is a primary driver of AChR antibody-positive MG (AChR+ MG) pathogenesis. This leads to the formation of the membrane attack complex and destruction of AChR receptors and the postsynaptic membrane resulting in impaired neurotransmission and muscle weakness characteristic of MG. Broad-based immune-suppressants like corticosteroids are effective in controlling MG; however, their long-term use can be associated with significant adverse effects. Advances in translational research have led to the development of more directed therapeutic agents that are likely to alter the future of MG treatment. Eculizumab is a humanized monoclonal antibody that inhibits the cleavage of complement protein C5 and is approved for use in generalized MG. In this review, we discuss the pathophysiology of MG; the therapeutic efficacy and tolerability of eculizumab, as well as the practical guidelines for its use in MG; future studies exploring the role of eculizumab in different stages and subtypes of MG subtypes; the optimal duration of therapy and its discontinuation; the characterization of non-responder patients; and the use of biomarkers for monitoring therapy are highlighted. Based on the pathophysiologic mechanisms, emerging therapies and new therapeutic targets are also reviewed.
Keywords: autoantibodies; complement; eculizumab; myasthenia gravis; pathophysiology.
© 2022 Waheed et al.
Conflict of interest statement
Professor Rup Tandan reports grants, personal fees from Alexion Pharmaceuticals, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

