Identification of a Novel Pyroptosis-Related Gene Signature Indicative of Disease Prognosis and Treatment Response in Skin Cutaneous Melanoma
- PMID: 35855761
- PMCID: PMC9288220
- DOI: 10.2147/IJGM.S367693
Identification of a Novel Pyroptosis-Related Gene Signature Indicative of Disease Prognosis and Treatment Response in Skin Cutaneous Melanoma
Abstract
Purpose: Pyroptosis plays an important role in the occurrence and progression of many tumors; however, the specific mechanisms involved remain unknown. Here, we construct a pyroptosis-related gene signature that can be used to predict survival prognosis of skin cutaneous melanoma (SKCM) and provide guidance for clinical treatment.
Methods: By integrating data from the two databases from the GTEx and TCGA, differentially expressed genes (DEGs) from normal tissues and skin cutaneous tumor tissues were identified. The main signaling pathways and function enrichment of these differential genes were determined. Univariate and multivariate COX regression analysis, and risk score analysis were used to construct a signature to assess its predictive value for overall survival. The mRNA expression of these five genes in melanoma cells was determined by quantitative polymerase chain reaction (qPCR). The pRRophetic algorithm was used to estimate the half-maximal inhibitory concentration (IC50) of chemotherapy drugs in SKCM patients. The expression of multiple immune checkpoint genes also was evaluated.
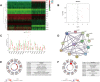
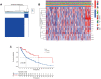
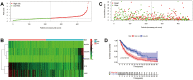
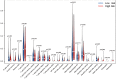
Results: Sixteen DEGs associated with pyroptosis in SKCM and normal skin tissues were identified. Of these, 12 pyroptosis-related DEGs were associated with the prognosis of SKCM. A five-gene signature (GSDMA, GSDMC, IL-18, NLRP6, and AIM2) model was constructed. Patients were divided into high-risk and low-risk groups using the risk scores. Of these, the high-risk group had a worse survival prognosis. There are significant differences in the predicted sensitivity of the high-risk and low-risk groups to chemotherapeutic drugs. In addition, compared with the high-risk group, the low-risk group showed higher expression of PD-1, PDL-1, CTLA-4, LAG-3, and VSIR.
Conclusion: In this study, we constructed a novel prognostic pyroptosis-related gene-signature for SKCM. These genes showed good predictive value for patient prognosis and could provide guidance for better treatment of SKCM patients.
Keywords: prognosis; pyroptosis; risk model; signature; skin cutaneous melanoma.
© 2022 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
System analysis based on the pyroptosis-related genes identifes GSDMD as a novel therapy target for skin cutaneous melanoma.J Transl Med. 2023 Nov 10;21(1):801. doi: 10.1186/s12967-023-04513-9. J Transl Med. 2023. PMID: 37950289 Free PMC article.
-
Construction and validation of a novel pyroptosis-related signature to predict prognosis in patients with cutaneous melanoma.Math Biosci Eng. 2022 Jan;19(1):688-706. doi: 10.3934/mbe.2022031. Epub 2021 Nov 19. Math Biosci Eng. 2022. PMID: 34903008
-
Identification of a pyroptosis-associated long non-coding RNA signature for predicting the immune status and prognosis in skin cutaneous melanoma.Eur Rev Med Pharmacol Sci. 2021 Sep;25(18):5597-5609. doi: 10.26355/eurrev_202109_26779. Eur Rev Med Pharmacol Sci. 2021. PMID: 34604952
-
The Landscape of the Tumor Microenvironment in Skin Cutaneous Melanoma Reveals a Prognostic and Immunotherapeutically Relevant Gene Signature.Front Cell Dev Biol. 2021 Oct 1;9:739594. doi: 10.3389/fcell.2021.739594. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34660598 Free PMC article.
-
Aspartate beta-hydroxylase domain containing 1 as a prognostic marker associated with immune infiltration in skin cutaneous melanoma.BMC Cancer. 2023 Mar 31;23(1):292. doi: 10.1186/s12885-023-10625-8. BMC Cancer. 2023. PMID: 37004045 Free PMC article.
Cited by
-
The Therapeutic Potential of Pyroptosis in Melanoma.Int J Mol Sci. 2023 Jan 9;24(2):1285. doi: 10.3390/ijms24021285. Int J Mol Sci. 2023. PMID: 36674798 Free PMC article. Review.
-
Multiomics characterization of pyroptosis in the tumor microenvironment and therapeutic relevance in metastatic melanoma.BMC Med. 2024 Jan 17;22(1):24. doi: 10.1186/s12916-023-03175-0. BMC Med. 2024. PMID: 38229080 Free PMC article.
-
Integrated transcriptomic and immunological profiling reveals new diagnostic and prognostic models for cutaneous melanoma.Front Pharmacol. 2024 May 28;15:1389550. doi: 10.3389/fphar.2024.1389550. eCollection 2024. Front Pharmacol. 2024. PMID: 38863979 Free PMC article.
-
Characteristics and significance of programmed cell death-related gene expression signature in skin cutaneous melanoma.Skin Res Technol. 2024 May;30(5):e13739. doi: 10.1111/srt.13739. Skin Res Technol. 2024. PMID: 38766879 Free PMC article.
-
The Efficacy of Electrochemotherapy with Dacarbazine on Melanoma Cells.Bioelectricity. 2024 Jun 12;6(2):118-125. doi: 10.1089/bioe.2023.0041. eCollection 2024 Jun. Bioelectricity. 2024. PMID: 39119570 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

