Activation of SIRT1 Alleviates Ferroptosis in the Early Brain Injury after Subarachnoid Hemorrhage
- PMID: 35855863
- PMCID: PMC9288286
- DOI: 10.1155/2022/9069825
Activation of SIRT1 Alleviates Ferroptosis in the Early Brain Injury after Subarachnoid Hemorrhage
Abstract
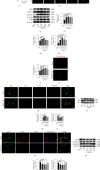
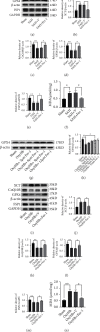
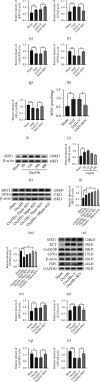
Ferroptosis is a regulated cell death that characterizes the lethal lipid peroxidation and iron overload, which may contribute to early brain injury (EBI) pathogenesis after subarachnoid hemorrhage (SAH). Although Sirtuin 1 (SIRT1), a class III histone deacetylase, has been proved to have endogenous neuroprotective effects on the EBI following SAH, the role of SIRT1 in ferroptosis has not been studied. Hence, we designed the current study to determine the role of ferroptosis in the EBI and explore the correlation between SIRT1 and ferroptosis after SAH. The pathways of ferroptosis were examined after experimental SAH in vivo (prechiasmatic cistern injection mouse model) and in HT-22 cells stimulated by oxyhemoglobin (oxyHb) in vitro. Then, ferrostatin-1 (Fer-1) was used further to determine the role of ferroptosis in EBI. Finally, we explored the correlation between SIRT1 and ferroptosis via regulating the expression of SIRT1 by resveratrol (RSV) and selisistat (SEL). Our results showed that ferroptosis was involved in the pathogenesis of EBI after SAH through multiple pathways, including acyl-CoA synthetase long-chain family member 4 (ACSL4) activation, iron metabolism disturbance, and the downregulation of glutathione peroxidase 4 (GPX4) and ferroptosis suppressor protein 1 (FSP1). Inhibition of ferroptosis by Fer-1 significantly alleviated oxidative stress-mediated brain injury. SIRT1 activation could suppress SAH-induced ferroptosis by upregulating the expression of GPX4 and FSP1. Therefore, ferroptosis could be a potential therapeutic target for SAH, and SIRT1 activation is a promising method to inhibit ferroptosis.
Copyright © 2022 Bin Yuan et al.
Conflict of interest statement
The authors declared that there is no conflict of interest regarding the publication of this paper.
Figures



References
-
- Rahman M. M., Islam F., Parvez A., et al. Citrus limon L. (lemon) seed extract shows neuro-modulatory activity in an in vivo thiopental-sodium sleep model by reducing the sleep onset and enhancing the sleep duration. Journal of Integrative Neuroscience . 2022;21(1):p. 42. doi: 10.31083/j.jin2101042. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

