lncRNA PINK1-AS Aggravates Cerebral Ischemia/Reperfusion Oxidative Stress Injury through Regulating ATF2 by Sponging miR-203
- PMID: 35855866
- PMCID: PMC9288285
- DOI: 10.1155/2022/1296816
lncRNA PINK1-AS Aggravates Cerebral Ischemia/Reperfusion Oxidative Stress Injury through Regulating ATF2 by Sponging miR-203
Abstract
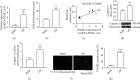
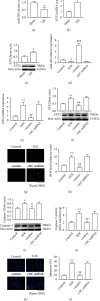
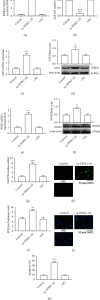
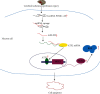
Ischemic stroke is a common disease that led to high mortality and high disability. NADPH oxidase 2- (NOX2-) mediated oxidative stress and long noncoding RNA have important roles in cerebral ischemia/reperfusion (CI/R) injury, whereas whether there is interplay between them remains to be clarified. This study was performed to observe the role of lncRNA PINK1-antisense RNA (PINK1-AS) in NOX2 expression regulation. An in vivo rat model (MCAO) and an in vitro cell model (H/R: hypoxia/reoxygenation) were utilized for CI/R oxidative stress injury investigation. The expression levels of lncRNA PINK1-AS, activating transcription factor 2 (ATF2), NOX2, and caspase-3 and the production level of ROS and cell apoptosis were significantly increased in CI/R injury model rats or in H/R-induced SH-SY5Y cells, but miR-203 was significantly downregulated. There was positive correlation between PINK1-AS expression level and ROS production level. PINK1-AS and ATF2 were found to be putative targets of miR-203. Knockdown of lncRNA PINK1-AS or ATF2 or the overexpression of miR-203 significantly reduced oxidative stress injury via inhibition of NOX2. Overexpression of lncRNA PINK1 significantly led to oxidative stress injury in SH-SY5Y cells through downregulating miR-203 and upregulating ATF2 and NOX2. lncRNA PINK1-AS and ATF2 were the targets of miR-203, and the lncRNA PINK1-AS/miR-203/ATF2/NOX2 axis plays pivotal roles in CI/R injury. Therefore, lncRNA PINK1-AS is a possible target for CR/I injury therapy by sponging miR-203.
Copyright © 2022 Zhong-Bao Yang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2.Biomed Pharmacother. 2020 Apr;124:109860. doi: 10.1016/j.biopha.2020.109860. Epub 2020 Jan 27. Biomed Pharmacother. 2020. PMID: 32000043
-
Long non-coding RNA NORAD protects against cerebral ischemia/reperfusion injury induced brain damage, cell apoptosis, oxidative stress and inflammation by regulating miR-30a-5p/YWHAG.Bioengineered. 2021 Dec;12(2):9174-9188. doi: 10.1080/21655979.2021.1995115. Bioengineered. 2021. PMID: 34709972 Free PMC article.
-
Long noncoding RNA SOX2OT silencing alleviates cerebral ischemia-reperfusion injury via miR-135a-5p-mediated NR3C2 inhibition.Brain Res Bull. 2021 Aug;173:193-202. doi: 10.1016/j.brainresbull.2021.05.018. Epub 2021 May 20. Brain Res Bull. 2021. PMID: 34022287
-
MicroRNAs Regulate Mitochondrial Function in Cerebral Ischemia-Reperfusion Injury.Int J Mol Sci. 2015 Oct 20;16(10):24895-917. doi: 10.3390/ijms161024895. Int J Mol Sci. 2015. PMID: 26492239 Free PMC article. Review.
-
Emerging Treatment Strategies for Cerebral Ischemia-Reperfusion Injury.Neuroscience. 2022 Dec 15;507:112-124. doi: 10.1016/j.neuroscience.2022.10.020. Epub 2022 Oct 28. Neuroscience. 2022. PMID: 36341725 Review.
Cited by
-
The Role of microRNAs in Gene Expression and Signaling Response of Tumor Cells to an Acidic Environment.Int J Mol Sci. 2023 Nov 29;24(23):16919. doi: 10.3390/ijms242316919. Int J Mol Sci. 2023. PMID: 38069241 Free PMC article.
-
Expression profile of long non-coding RNA in inner Mongolian cashmere goat with putative roles in hair follicles development.Front Vet Sci. 2022 Sep 2;9:995604. doi: 10.3389/fvets.2022.995604. eCollection 2022. Front Vet Sci. 2022. PMID: 36118352 Free PMC article.
-
Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke.Antioxidants (Basel). 2022 Nov 30;11(12):2377. doi: 10.3390/antiox11122377. Antioxidants (Basel). 2022. PMID: 36552584 Free PMC article. Review.
-
LncRNAs and CircRNAs as Strategies against Pathological Conditions Caused by a Hypoxic/Anoxic State.Biomolecules. 2023 Nov 6;13(11):1622. doi: 10.3390/biom13111622. Biomolecules. 2023. PMID: 38002304 Free PMC article. Review.
-
Effects of ATF2/TSC1 on epilepsy by modulating the microphages polarization of microglia.Sci Rep. 2025 Jul 2;15(1):22958. doi: 10.1038/s41598-025-04914-4. Sci Rep. 2025. PMID: 40595935 Free PMC article.
References
-
- Jackman K. A., Miller A. A., De Silva T. M., Crack P. J., Drummond G. R., Sobey C. G. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. British Journal of Pharmacology . 2009;156(4):680–688. doi: 10.1111/j.1476-5381.2008.00073.x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

