Long-Term Effect of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Injections in Choroidal Neovascularization Secondary to Angioid Streaks
- PMID: 35855887
- PMCID: PMC9288306
- DOI: 10.1155/2022/3332421
Long-Term Effect of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Injections in Choroidal Neovascularization Secondary to Angioid Streaks
Abstract
Purpose: This study aimed to evaluate the long-term effectiveness of intravitreal anti-vascular endothelial growth factor (VEGF) injections in the treatment of choroidal neovascularization (CNV) associated with angioid streaks.
Methods: Multicenter retrospective cohort study, including eyes with CNV secondary to angioid streaks treated with anti-VEGF injections, were performed. Best-corrected visual acuity (BCVA) in ETDRS letters; qualitative and quantitative (foveal thickness) OCT parameters; anti-VEGF type; and number of injections were collected at baseline and at 3, 6, 12, 24, 36, 48, 60, and 72 months.
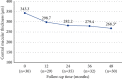
Results: Thirty-nine eyes from 29 patients, 17 (58.6%) females, were included. The mean follow-up time was 69.4 ± 34.5 months. BCVA was 59.3 ± 23.3 letters at baseline and 63.7 ± 21.9 letters at 48 months. At 3 months, BCVA improved 6.9 ± 11.7 letters (P=0.003). Then, BCVA remained stable. The mean foveal thickness decreased from 343.3 ± 120.2 μm at baseline to 268.3 ± 65.4 at 48 months (P=0.021). The mean number of injections was 4.6 ± 2.1 at 12 months, decreasing to 1.7 ± 2.4 injections between 36 and 48 months (P=0.093).
Conclusion: This real-world study suggests that the functional and morphologic response to anti-VEGF therapy for CNV related to angioid streaks is generally satisfactory and maintained in the long term.
Copyright © 2022 Sónia Torres-Costa et al.
Conflict of interest statement
Manuel Falcão has participated in advisory boards for Bayer and has received travel grants from Novartis, Alimera, and Allergan. Ângela Carneiro has participated in advisory boards for Alcon, Bayer, Novartis, Alimera, Allergan, and Roche. The other authors report no conflicts of interest in this work.
Figures


Similar articles
-
Long-term follow-up of management of choroidal neovascularisation secondary to angioid streaks with intravitreal anti-vascular endothelial growth factor.Eye (Lond). 2021 Mar;35(3):853-857. doi: 10.1038/s41433-020-0979-9. Eye (Lond). 2021. PMID: 32461565 Free PMC article.
-
Intravitreal Bevacizumab for Nonsubfoveal Choroidal Neovascularization Associated With Angioid Streaks: 3-Year Follow-up Study.Am J Ophthalmol. 2016 May;165:174-8. doi: 10.1016/j.ajo.2016.03.017. Epub 2016 Mar 21. Am J Ophthalmol. 2016. PMID: 27013066
-
Intravitreal bevacizumab for the treatment of choroidal neovascularization secondary to angioid streaks: one year of follow-up.Acta Ophthalmol. 2011 Nov;89(7):641-6. doi: 10.1111/j.1755-3768.2009.01836.x. Epub 2010 Dec 14. Acta Ophthalmol. 2011. PMID: 21155980
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD009510. doi: 10.1002/14651858.CD009510.pub3. Cochrane Database Syst Rev. 2020. PMID: 32633861 Free PMC article.
-
Treatment of choroidal neovascularization due to angioid streaks: a comprehensive review.Retina. 2013 Jul-Aug;33(7):1300-14. doi: 10.1097/IAE.0b013e3182914d2b. Retina. 2013. PMID: 23719398 Review.
Cited by
-
En-Face Optical Coherence Tomography Is Useful for Assessing Striated Lesions in Angioid Streaks: A Case Report.Cureus. 2023 Sep 26;15(9):e45983. doi: 10.7759/cureus.45983. eCollection 2023 Sep. Cureus. 2023. PMID: 37900525 Free PMC article.
-
Long-term functional, anatomical outcome, and qualitative analysis by OCTA, as a predictor of disease recurrences in patients with choroidal neovascularization secondary to angioid streaks.Int J Retina Vitreous. 2024 Jul 29;10(1):53. doi: 10.1186/s40942-024-00568-y. Int J Retina Vitreous. 2024. PMID: 39075569 Free PMC article.
-
A Case Report of Choroidal Neovascularization Secondary to Angioid Streaks Associated With Beta Thalassemia.Cureus. 2025 May 25;17(5):e84800. doi: 10.7759/cureus.84800. eCollection 2025 May. Cureus. 2025. PMID: 40568266 Free PMC article.
-
Angioid Streaks Remain a Challenge in Diagnosis, Management, and Treatment.Vision (Basel). 2024 Mar 5;8(1):10. doi: 10.3390/vision8010010. Vision (Basel). 2024. PMID: 38535759 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

