Ionogels Based on a Single Ionic Liquid for Electronic Nose Application
- PMID: 35855953
- PMCID: PMC7613049
- DOI: 10.3390/chemosensors9080201
Ionogels Based on a Single Ionic Liquid for Electronic Nose Application
Abstract
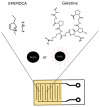
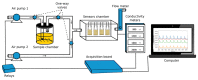
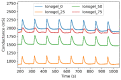
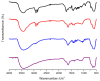
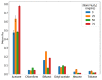
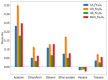
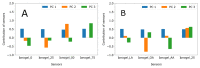
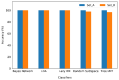
Ionogel are versatile materials, as they present the electrical properties of ionic liquids and also dimensional stability, since they are trapped in a solid matrix, allowing application in electronic devices such as gas sensors and electronic noses. In this work, ionogels were designed to act as a sensitive layer for the detection of volatiles in a custom-made electronic nose. Ionogels composed of gelatin and a single imidazolium ionic liquid were doped with bare and functionalized iron oxide nanoparticles, producing ionogels with adjustable target selectivity. After exposing an array of four ionogels to 12 distinct volatile organic compounds, the collected signals were analyzed by principal component analysis (PCA) and by several supervised classification methods, in order to assess the ability of the electronic nose to distinguish different volatiles, which showed accuracy above 98%.
Keywords: composite; electronic nose; gas sensor; ionic liquid; ionogel; volatile organic compound.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures


References
-
- Ghandi K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain Chem. 2014;4:44–53. doi: 10.4236/gsc.2014.41008. - DOI
-
- Keaveney ST, Haines RS, Harper JB. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure Appl Chem. 2017;89:745–757. doi: 10.1515/pac-2016-1008. - DOI
-
- Zolfigol MA, Khazaei A, Moosavi-Zare AR, Zare A. 3-Methyl-1-sulfonic acid imidazolium chloride as a new, Efficient and recyclable catalyst and solvent for the preparation of N-sulfonyl imines at room Temperature. J Iran Chem Soc. 2010;7:646–651. doi: 10.1007/BF03246053. - DOI
-
- Galiński M, Lewandowski A, Stepniak I. Ionic liquids as electrolytes. Electrochim Acta. 2006;51:5567–5580. doi: 10.1016/j.electacta.2006.03.016. - DOI
-
- Sasikumar B, Arthanareeswaran G, Ismail AF. Recent progress in ionic liquid membranes for gas separation. J Mol Liq. 2018;266:330–341. doi: 10.1016/j.molliq.2018.06.081. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
