Guillain-Barré syndrome following SARS-CoV-2 vaccination in the UK: a prospective surveillance study
- PMID: 35856053
- PMCID: PMC9277028
- DOI: 10.1136/bmjno-2022-000309
Guillain-Barré syndrome following SARS-CoV-2 vaccination in the UK: a prospective surveillance study
Abstract
Objective: To investigate features of Guillain-Barré syndrome (GBS) following SARS-CoV-2 vaccines and evaluate for a causal link between the two.
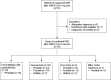
Methods: We captured cases of GBS after SARS-CoV-2 vaccination through a national, open-access, online surveillance system. For each case, the certainty of GBS was graded using the Brighton criteria, and the relationship to the vaccine was examined using modified WHO Causality Assessment criteria. We compared age distribution of cases with that of prepandemic GBS cases and clinical features with the International GBS Outcome Study (IGOS).
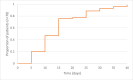
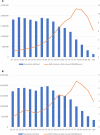
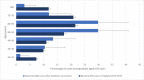
Results: Between 1 January and 30 June 2021, we received 67 reports of GBS following the ChAdOx1 vaccine (65 first doses) and three reports following the BNT162b2 vaccine (all first doses). The causal association with the vaccine was classified as probable for 56 (80%, all ChAdOx1), possible for 12 (17%, 10 ChAdOx1) and unlikely for two (3%, 1 ChAdOx1). A greater proportion of cases occurred in the 50-59 age group in comparison with prepandemic GBS. Most common clinical variants were sensorimotor GBS (n=55; 79%) and facial diplegia with paraesthesias (n=10; 14%). 10% (n=7/69) of patients reported an antecedent infection, compared with 77% (n=502/652) of the IGOS cohort (p<0.00001). Facial weakness (63% (n=44/70) vs 36% (n=220/620); p<0.00001) and sensory dysfunction (93% (n=63/68) vs 69% (n=408/588); p=0.00005) were more common but disease severity and outcomes were similar to the IGOS study.
Interpretation: Most reports of GBS followed the first dose of ChAdOx1 vaccine. While our study cannot confirm or refute causation, this observation, together with the absence of alternative aetiologies, different than expected age distribution and the presence of unusual clinical features support a causal link. Clinicians and surveillance bodies should remain vigilant to the possibility of this very rare adverse event and its atypical variants.
Keywords: COVID-19; clinical neurology; guillain-barre syndrome.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: TS was chair/cochair of the UK Research and Innovation/National Institute for Health Research COVID-19 Rapid Response and Rolling Funding Initiatives, was an Advisor to the UK COVID-19 Therapeutics Advisory Panel and is a member of the UK Medicines and Healthcare Products Regulatory Agency COVID-19 Vaccines Benefit Risk Expert Working Group. BCJ is a chair of Steering Committee of IGOS. HM is an invited expert for the Commission on Human Medicines COVID-19 Vaccines Safety Surveillance Methodologies Expert Working Group. The remaining authors have no relevant conflict of interest to declare.
Figures
References
-
- GOV.UK . Vaccinations in the UK, 2021. Available: https://coronavirus.data.gov.uk/details/vaccinations
-
- GOV.UK . COVID-19 vaccination first phase priority groups, 2021. Available: https://www.gov.uk/government/publications/covid-19-vaccination-care-hom...
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
