Photoactivation Properties of Self-n-Doped Perylene Diimides: Concentration-dependent Radical Anion and Dianion Formation
- PMID: 35856074
- PMCID: PMC9284616
- DOI: 10.1021/acsmaterialsau.2c00019
Photoactivation Properties of Self-n-Doped Perylene Diimides: Concentration-dependent Radical Anion and Dianion Formation
Abstract
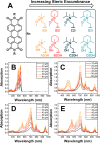
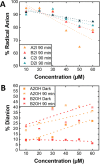
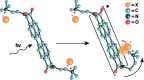
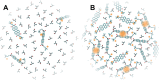
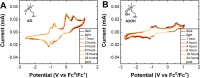
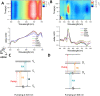
Perylene diimides (PDIs) have garnered attention as organic photocatalysts in recent years for their ability to drive challenging synthetic transformations, such as aryl halide reduction and olefin iodoperfluoroalkylation. Previous work in this area employs spectator pendant groups attached to the imide nitrogen positions of PDIs that are only added to impart solubility. In this work, we employ electron-rich ammonium iodide or ammonium hydroxide pendant groups capable of self-n-doping the PDI core to form radical anions (R •- ) and dianions (D ••2- ). We observe R •- formation is favored at low concentrations where aliphatic linkers are able to freely rotate, while D ••2- formation is favored at elevated concentrations likely due to Coulombic stabilization between adjacent chromophores in a similar manner to that of Kasha exciton stabilization. Cyclic voltammetric measurements are consistent with steric encumbrance increasing the Lewis basicity of anions through Coulombic destabilization. However, sterics also inhibit dianion formation by disrupting aggregation. Finally, femtosecond transient absorption measurements reveal that low wavelength excitation (400 nm) preferentially favors the excitation of R •- to the strongly reducing doublet excited state 2[R •- ]*. In contrast, higher wavelength excitation (520 nm) favors the formation of the singlet excited state 1[N]*. These findings highlight the importance of dopant architecture, counterion selection, excitation wavelength, and concentration on R •- and D ••2- formation, which has substantial implications for future photocatalytic applications. We anticipate these findings will enable more efficient systems based on self-n-doped PDIs.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Wang Z.; Zheng N.; Zhang W.; Yan H.; Xie Z.; Ma Y.; Huang F.; Cao Y. Self-Doped, n-Type Perylene Diimide Derivatives as Electron Transporting Layers for High-Efficiency Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1700232.10.1002/aenm.201700232. - DOI
-
- Ronconi F.; Syrgiannis Z.; Bonasera A.; Prato M.; Argazzi R.; Caramori S.; Cristino V.; Bignozzi C. A. Modification of Nanocrystalline WO3 with a Dicationic Perylene Bisimide: Applications to Molecular Level Solar Water Splitting. J. Am. Chem. Soc. 2015, 137, 4630–4633. 10.1021/jacs.5b01519. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
