β-hydroxybutyrate reduces blastocyst viability via trophectoderm-mediated metabolic aberrations in mice
- PMID: 35856159
- PMCID: PMC9433850
- DOI: 10.1093/humrep/deac153
β-hydroxybutyrate reduces blastocyst viability via trophectoderm-mediated metabolic aberrations in mice
Abstract
Study question: What is the effect of the ketone β-hydroxybutyrate (βOHB) on preimplantation mouse embryo development, metabolism, epigenetics and post-transfer viability?
Summary answer: In vitro βOHB exposure at ketogenic diet (KD)-relevant serum concentrations significantly impaired preimplantation mouse embryo development, induced aberrant glycolytic metabolism and reduced post-transfer fetal viability in a sex-specific manner.
What is known already: A maternal KD in humans elevates gamete and offspring βOHB exposure during conception and gestation, and in rodents is associated with an increased time to pregnancy, and altered offspring organogenesis, post-natal growth and behaviour, suggesting a developmental programming effect. In vitro exposure to βOHB at supraphysiological concentrations (8-80 mM) perturbs preimplantation mouse embryo development.
Study design, size, duration: A mouse model of embryo development and viability was utilized for this laboratory-based study. Embryo culture media were supplemented with βOHB at KD-relevant concentrations, and the developmental competence, physiology, epigenetic state and post-transfer viability of in vitro cultured βOHB-exposed embryos was assessed.
Participants/materials, setting, methods: Mouse embryos were cultured in vitro with or without βOHB at concentrations representing serum levels during pregnancy (0.1 mM), standard diet consumption (0.25 mM), KD consumption (2 mM) and diabetic ketoacidosis (4 mM). The impact of βOHB exposure on embryo development (blastocyst formation rate, morphokinetics and blastocyst total, inner cell mass and trophectoderm (TE) cell number), physiology (redox state, βOHB metabolism, glycolytic metabolism), epigenetic state (histone 3 lysine 27 β-hydroxybutyrylation, H3K27bhb) and post-transfer viability (implantation rate, fetal and placental development) was assessed.
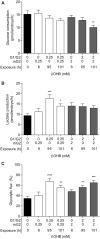
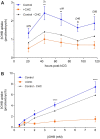
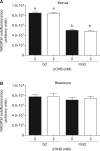
Main results and the role of chance: All βOHB concentrations tested slowed embryo development (P < 0.05), and βOHB at KD-relevant serum levels (2 mM) delayed morphokinetic development, beginning at syngamy (P < 0.05). Compared with unexposed controls, βOHB exposure reduced blastocyst total and TE cell number (≥0.25 mM; P < 0.05), reduced blastocyst glucose consumption (2 mM; P < 0.01) and increased lactate production (0.25 mM; P < 0.05) and glycolytic flux (0.25 and 2 mM; P < 0.01). Consumption of βOHB by embryos, mediated via monocarboxylate transporters, was detected throughout preimplantation development. Supraphysiological (20 mM; P < 0.001), but not physiological (0.25-4 mM) βOHB elevated H3K27bhb levels. Preimplantation βOHB exposure at serum KD levels (2 mM) reduced post-transfer viability. Implantation and fetal development rates of βOHB-treated embryos were 50% lower than controls (P < 0.05), and resultant fetuses had a shorter crown-rump length (P < 0.01) and placental diameter (P < 0.05). A strong sex-specific effect of βOHB was detected, whereby female fetuses from βOHB-treated embryos weighed less (P < 0.05), had a shorter crown-rump length (P < 0.05), and tended to have accelerated ear development (P < 0.08) compared with female control fetuses.
Limitations, reasons for caution: This study only assessed embryo development, physiology and viability in a mouse model utilizing in vitro βOHB exposure; the impact of in vivo exposure was not assessed. The concentrations of βOHB utilized were modelled on blood/serum levels as the true oviduct and uterine concentrations are currently unknown.
Wider implications of the findings: These findings indicate that the development, physiology and viability of mouse embryos is detrimentally impacted by preimplantation exposure to βOHB within a physiological range. Maternal diets which increase βOHB levels, such as a KD, may affect preimplantation embryo development and may therefore impair subsequent viability and long-term health. Consequently, our initial observations warrant follow-up studies in larger human populations. Furthermore, analysis of βOHB concentrations within human and rodent oviduct and uterine fluid under different nutritional states is also required.
Study funding/competing interest(s): This work was funded by the University of Melbourne and the Norma Hilda Schuster (nee Swift) Scholarship. The authors have no conflicts of interest.
Trial registration number: N/A.
Keywords: DOHaD; beta-hydroxybutyrylation; embryo transfer; epigenetics; ketogenic diet; ketone; metabolism; morphokinetics; nutrients.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures


References
-
- Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T.. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod 2011;26:3289–3296. - PubMed
-
- Alwahab UA, Pantalone KM, Burguera B.. A ketogenic diet may restore fertility in women with polycystic ovary syndrome: a case series. AACE Clin Case Rep 2018;4:e427–e431.
-
- Barker DJ, Osmond C.. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986;327:1077–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

