Work productivity in real-life employed patients with plaque psoriasis: Results from the ProLOGUE study
- PMID: 35856276
- PMCID: PMC9796840
- DOI: 10.1111/1346-8138.16517
Work productivity in real-life employed patients with plaque psoriasis: Results from the ProLOGUE study
Abstract
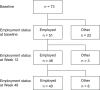
Psoriasis poses a substantial economic burden by reducing the work productivity of affected patients. We aimed to evaluate the negative impact of plaque psoriasis on work productivity and effectiveness of brodalumab in improving work productivity impairment in real-life employed patients. This analysis was conducted in employed patients from ProLOGUE, an open-label, multicenter, prospective cohort study (Japan Registry of Clinical Trials identifier: jRCTs031180037). Outcomes included association of Work Productivity and Activity Impairment-Psoriasis (WPAI-PSO) domain scores with scores from various patient-reported outcome measures or Psoriasis Area and Severity Index (PASI) scores at baseline. Change from baseline in WPAI-PSO domain scores following brodalumab treatment was also evaluated. Of the 73 patients enrolled, 51, 48, and 40 patients were considered employed at baseline, Week 12, and Week 48 of brodalumab treatment, respectively. In the model adjusted by age and sex, the work productivity loss score correlated with the Dermatology Life Quality Index (DLQI), itch Numeric Rating Scale (NRS), Patient Health Questionnaire-8 (PHQ-8), and skin pain NRS scores (partial Spearman correlation coefficient [ρ] = 0.608, 0.510, 0.461, and 0.424, respectively); presenteeism score correlated with the DLQI, itch NRS, and skin pain NRS scores (ρ = 0.568, 0.500, and 0.403, respectively); and activity impairment score correlated with the DLQI and PHQ-8 scores (ρ = 0.530 and 0.414, respectively). None of the WPAI-PSO domain scores correlated with the PASI score. All WPAI-PSO domain scores (except absenteeism) significantly reduced from baseline to Weeks 12 (p < 0.0001) and 48 (p < 0.001) with brodalumab treatment. In conclusion, work productivity impairment in psoriasis was associated with various subjective symptoms that can be captured using patient-reported outcome measures. Brodalumab treatment improved work productivity in real-life employed patients with plaque psoriasis.
Keywords: brodalumab; employment; patient-reported outcome measures; psoriasis; work performance.
© 2022 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
H. Saeki reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from Kyowa Kirin, Mitsubishi Tanabe Pharma, Taiho Pharmaceutical, Maruho, TOKIWA Pharmaceutical, Torii Pharmaceutical, and Eisai outside the submitted work; and personal fees and nonfinancial support from Sanofi, Celgene, and KYORIN Pharmaceutical outside the submitted work. Y. Kanai is an employee of Kyowa Kirin. K. Murotani reports grants from Kyowa Kirin during the study period. K. Ito reports grants from Kyowa Kirin during the study period and personal fees from Kyowa Kirin, Mitsubishi Tanabe Pharma, Sato Pharmaceutical, Ushio, Amgen, Janssen Pharmaceutical, AbbVie, Eisai, Sanofi, Eli Lilly Japan, Maruho, Nippon Kayaku, Taiho Pharmaceutical, and Novartis Pharma outside the submitted work. T. Miyagi reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from AbbVie, Kaken Pharmaceutical, Maruho, Boehringer Ingelheim, Eisai, Celgene, Eli Lilly Japan, Novartis Pharma, and Taiho Pharmaceutical outside the submitted work; grants and personal fees from Daiichi Sankyo, Sanofi, Ono Pharmaceutical, Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceutical; grants from Actelion, Earth Corporation, Teijin Pharma, LEO Pharma, and Sato Pharmaceutical outside the submitted work; and personal fees from CSL Behring outside the submitted work. H. Takahashi reports grants from Kyowa Kirin during the study period. Y. Tada reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from Kyowa Kirin, Eli Lilly Japan, AbbVie, Maruho, Celgene, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Sanofi, UCB Japan, Torii Pharmaceutical, LEO Pharma, Eisai, Kaken Pharmaceutical, Pfizer, Ushio, Meiji Seika Pharma, Nippon Boehringer Ingelheim, JIMRO, Bristol Myers Squibb, and TOKIWA Pharmaceutical outside the submitted work; grants and nonfinancial support from Kanebo Cosmetics, MSD, Ono Pharmaceutical, Pola Pharma, Nihon Pharmaceutical, Smith & Nephew, and Sato Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceutical outside the submitted work; grants from Japan Blood Products Organization, Mochida Healthcare, Oshimatsubaki, and Shionogi outside the submitted work; and personal fees from Chugai Pharmaceutical outside the submitted work. M. Higashiyama reports grants from Kyowa Kirin during the study period; personal fees and nonfinancial support from LEO Pharma outside the submitted work; and personal fees from Kyowa Kirin, AbbVie, Celgene, Taiho Pharmaceutical, Torii Pharmaceutical, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Novartis Pharma, Maruho, and Janssen Pharmaceutical outside the submitted work. Y. Hashimoto reports grants from Kyowa Kirin during the study period and personal fees from Kyowa Kirin, Eisai, AbbVie, Eli Lilly Japan, Nippon Kayaku, Janssen Pharmaceutical, Taiho Pharmaceutical, Torii Pharmaceutical, LEO Pharma, Maruho, UCB Japan, Novartis Pharma, and Celgene outside the submitted work. H. Kitabayashi is an employee of Kyowa Kirin and owns stock in the company. S. Imafuku reports grants from Kyowa Kirin during the study period; grants and personal fees from AbbVie, Eisai, Kaken Pharmaceutical, Kyowa Kirin, Sato Pharmaceutical, Sanofi, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Tsumura, Torii Pharmaceutical, Nippon Zoki Pharmaceutical, Novartis Pharma, Maruho, and LEO Pharma outside the submitted work; grants from Pola Pharma outside the submitted work; and personal fees from Astellas, Eli Lilly Japan, MSD, Otsuka Pharmaceutical, Ono Pharmaceutical, Sun Pharma, GSK, JIMRO, Celgene, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Japan Blood Products Organization, Pfizer, Bristol Myers Squibb, Meiji Seika Pharma, Janssen Pharmaceutical, and UCB Japan outside the submitted work.
Figures



Similar articles
-
Treatment with brodalumab is not associated with improved sleep problems in real-life patients with plaque psoriasis: Results of the ProLOGUE study.J Dermatol. 2023 Mar;50(3):319-326. doi: 10.1111/1346-8138.16620. Epub 2022 Nov 7. J Dermatol. 2023. PMID: 36342070
-
Effectiveness of brodalumab in improving itching and skin pain in Japanese patients with psoriasis: The ProLOGUE study.J Dermatol. 2023 Apr;50(4):453-461. doi: 10.1111/1346-8138.16682. Epub 2022 Dec 20. J Dermatol. 2023. PMID: 36540010
-
Utility of the Dermatology Life Quality Index at initiation or switching of biologics in real-life Japanese patients with plaque psoriasis: Results from the ProLOGUE study.J Dermatol Sci. 2021 Mar;101(3):185-193. doi: 10.1016/j.jdermsci.2021.01.002. Epub 2021 Jan 7. J Dermatol Sci. 2021. PMID: 33495058 Clinical Trial.
-
Brodalumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: An Evidence Review Group Evaluation of a NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Feb;37(2):131-139. doi: 10.1007/s40273-018-0698-2. Pharmacoeconomics. 2019. PMID: 30112635 Review.
-
Brodalumab: A Review in Moderate to Severe Plaque Psoriasis.Drugs. 2018 Mar;78(4):495-504. doi: 10.1007/s40265-018-0888-4. Drugs. 2018. PMID: 29516365 Review.
Cited by
-
A Systematic Review of 207 Studies Describing Validation Aspects of the Dermatology Life Quality Index.Acta Derm Venereol. 2024 Nov 7;104:adv41120. doi: 10.2340/actadv.v104.41120. Acta Derm Venereol. 2024. PMID: 39508500 Free PMC article.
-
Improvements in Psoriasis-Related Work Productivity with Tildrakizumab: Results from a Phase 4 Real-World Study in Patients with Moderate-to-Severe Plaque Psoriasis.Dermatol Ther (Heidelb). 2024 Apr;14(4):1019-1025. doi: 10.1007/s13555-024-01131-1. Epub 2024 Apr 4. Dermatol Ther (Heidelb). 2024. PMID: 38575729 Free PMC article.
-
Gasdermin A (GSDMA) Tissue Expression, Serum and Urinary Concentrations with Clinicopathologic Outcome in Psoriasis.Dermatol Pract Concept. 2024 Jul 1;14(3):e2024177. doi: 10.5826/dpc.1403a177. Dermatol Pract Concept. 2024. PMID: 39122519 Free PMC article.
-
Assessing the burden of dermatological diseases on work life from a gender perspective.Sci Rep. 2025 Jul 5;15(1):24014. doi: 10.1038/s41598-025-07804-x. Sci Rep. 2025. PMID: 40617854 Free PMC article.
References
-
- AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis ‐ comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59:566–71. - PubMed
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7. - PubMed
-
- Chen KC, Hung ST, Yang CW, Tsai TF, Tang CH. The economic burden of psoriatic diseases in Taiwan. J Dermatol Sci. 2014;75:183–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

