Sulfoxonium Ylides in Aminocatalysis: An Enantioselective Entry to Cyclopropane-Fused Chromanol Structures
- PMID: 35856291
- PMCID: PMC9344464
- DOI: 10.1021/acs.orglett.2c02204
Sulfoxonium Ylides in Aminocatalysis: An Enantioselective Entry to Cyclopropane-Fused Chromanol Structures
Abstract
The 1,1a,2,7b-tetrahydrocyclopropa[c]chromene, arising from fusion of chromane and cyclopropane rings is the core of medicinally relevant compounds. Engaging sulfoxonium ylides in enantioselective aminocatalytic reactions for the first time, a convenient entry to this scaffold is presented. Several ring-fused derivatives were obtained in moderate-to-good yields and enantioselectivities and with perfect diastereoselectivity at the cyclopropane, using an α,α-diphenylprolinol aminocatalyst. The versatility of the hemiacetal moiety in the products was leveraged to effect various synthetic manipulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

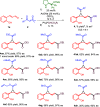
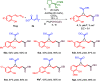

References
-
- Pallin T. D.; Cramp S. M.; Dyke H. J.; Zahler R. (Zafgen Inc.) Tricyclic compounds for use in the treatment and/or control of obesity. WO2014071369.
- Hughes T. E.; Vath J. E. (Zafgen Inc.) Methods of treating liver diseases. WO2014071368.
-
- Sahlberg C.; Zhou X.-X. Development of Non-Nucleoside Reverse Transcriptase Inhibitors for Anti-HIV Therapy. Antiinfect. Agents in Med. Chem. 2008, 7, 101–117. 10.2174/187152108783954597. - DOI
-
- Ge M.; He J.; Lau F. W. Y.; Liang G.-B.; Lin S.; Liu W.; Walsh S. P.; Yang L.. (Merck & Co. Inc.) Antidiabetic bicylic compounds. US20070265332.
-
- Asakawa Y.; Kondo K.; Tori M. Cyclopropanochroman derivatives from the liverwort Radula javanica. Phytochem. 1991, 30, 325–328. 10.1016/0031-9422(91)84147-K. - DOI
- Wang X.; Li L.; Zhu R.; Zhang J.; Zhou J.; Lou H. Bibenzyl-Based Meroterpenoid Enantiomers from the Chinese Liverwort Radula sumatrana. J. Nat. Prod. 2017, 80, 3143–3150. 10.1021/acs.jnatprod.7b00394. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

