FOXM1 network in association with TREM1 suppression regulates NET formation in diabetic foot ulcers
- PMID: 35856334
- PMCID: PMC9346470
- DOI: 10.15252/embr.202154558
FOXM1 network in association with TREM1 suppression regulates NET formation in diabetic foot ulcers
Abstract
Diabetic foot ulcers (DFU) are a serious complication of diabetes mellitus and associated with reduced quality of life and high mortality rate. DFUs are characterized by a deregulated immune response with decreased neutrophils due to loss of the transcription factor, FOXM1. Diabetes primes neutrophils to form neutrophil extracellular traps (NETs), contributing to tissue damage and impaired healing. However, the role of FOXM1 in priming diabetic neutrophils to undergo NET formation remains unknown. Here, we found that FOXM1 regulates reactive oxygen species (ROS) levels in neutrophils and inhibition of FOXM1 results in increased ROS leading to NET formation. Next generation sequencing revealed that TREM1 promoted the recruitment of FOXM1+ neutrophils and reversed effects of diabetes and promoted wound healing in vivo. Moreover, we found that TREM1 expression correlated with clinical healing outcomes of DFUs, indicating TREM1 may serve as a useful biomarker or a potential therapeutic target. Our findings highlight the clinical relevance of TREM1, and indicates FOXM1 pathway as a novel regulator of NET formation during diabetic wound healing, revealing new therapeutic strategies to promote healing in DFUs.
Keywords: FOXM1; TREM1; diabetic foot ulcers; neutrophil extracellular traps; neutrophils.
© 2022 The Authors. Published under the terms of the CC BY NC ND 4.0 license. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures

- A
Enriched GO processes from human skin acute day 3 wounds compared to human DFU demonstrates processes involved in neutrophil function to be deregulated in DFUs compared to acute wounds.
- B
Neutrophil gene signature comparing human skin acute day 3 wounds to human DFUs demonstrating decreased presence of neutrophils in human DFUs.
- C
qPCR validations of neutrophil genes. n = 7 DFUs and n = 3 skin acute day 3 wounds. **P < 0.01 (two‐tailed unpaired Student's t‐test). Data presented as mean ± SD.
- D
Ingenuity Pathway Analysis of predicted network shows activation of cell viability of neutrophils and inhibition of cell death of neutrophils in human skin wounds compared to activation of cell death of neutrophils in human DFUs.

- A
Representative images of human neutrophils treated with the FOXM1 inhibitor, FDI‐6. Vehicle (DMSO) served as a control. Neutrophils undergoing NET formation are visualized in green and live neutrophils are visualized in red. Quantification was performed by normalizing the number of neutrophils undergoing NET formation to the number of live neutrophils. Neutrophils from n = 3 different blood donors were isolated, pooled, and performed in triplicate. *P < 0.05 (two‐way ANOVA followed by Tukey's post‐hoc test). Data presented as mean ± SD. (Scale bar: 100 μm).
- B
Quantification of ROS levels in human neutrophils treated with FDI‐6 or in combination with N‐acetylcysteine (NAC). Phorbol 12‐myristate 13‐acetate (PMA) treatment served as a positive control. Data presented as mean ± SD. Neutrophils from n = 3 different blood donors were isolated, pooled and performed in triplicate. *P < 0.05 (two‐way ANOVA followed by Tukey's post‐hoc test).
- C
Quantification of NET formation in human neutrophils treated with FDI‐6 or in combination with NAC. PMA treatment served as a positive control. Neutrophils from n = 3 different blood donors were isolated, pooled, and performed in triplicate. Data presented as mean ± SD. *P < 0.05 (two‐way ANOVA followed by Tukey's post‐hoc test).
- A
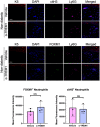
Human neutrophils cultured in either normal glucose media (5 mM glucose) or high glucose media (25 mM) to recapitulate diabetic conditions and treated with FDI‐6. Vehicle (DMSO) served as a control. NETs were visualized by immunostaining with citH3 and the neutrophil marker, elastase. (Scale bar: 50 μm). Quantification of n = 4 biological replicates per group were performed. Data presented as mean ± SD. *P < 0.05, and **P < 0.01 (one‐way ANOVA followed by Sidak post‐hoc test).
- B
Immunofluorescence staining of vehicle (DMSO) and FDI‐6 treated wounds in STZ‐induced diabetic mice at day 4 showing basal keratin marker K5, and NET marker citH3. Treatment of diabetic wounds with FDI‐6 resulted in increased citH3 compared to vehicle treated wounds. (Scale bar: 50 μm). Quantification of wound areas in n = 3 wounds per group were performed with Fiji software. Data presented as mean ± SD. **P < 0.01 (one‐way ANOVA followed by Sidak post‐hoc test).

- A
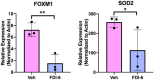
FOXM1 regulates genes involved in reducing ROS levels and is activated in human skin acute day 3 wounds compared to suppression in human DFUs.
- B
Representative images of human neutrophils treated with the FOXM1 inhibitor, FDI‐6, in presence or absence of NAC. PMA served as positive control. Reactive oxygen species formation are visualized in green. Representative images of human neutrophils treated with the FOXM1 inhibitor, FDI‐6, in presence or absence of NAC. PMA served as positive control. NET formation are visualized in green. (Scale bar: 100 μm).

- A
Upstream regulators found to be activated in human skin wounds that are suppressed or partially regulated in human DFUs involved in neutrophil response.
- B
TREM1 functions related to neutrophil response shows activation in human skin wounds compared to suppression in human DFUs.
- C
TREM1 predicted network connecting downstream target genes to their downstream biological processes leading to FOXM1 activation.

- A
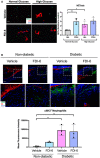
Representative images of wounded skin after topical treatment with either vehicle (IgG isotype control) or ⍺‐TREM1 activator at 0, 2, 4, 6, and 8 days after wounding. Percent of wound area at each time following vehicle or ⍺‐TREM1 activator treatment relative to the original wound area. Quantification of wound areas in n = 10 for vehicle diabetic and 12 wounds for ⍺‐TREM1‐treated wounds were performed with Fiji software. Data presented as mean ± SD. **P < 0.01, and ****P < 0.0001 (two‐way ANOVA followed by Tukey's post‐hoc test).
- B
Representative pictures of vehicle (IgG isotype control) and ⍺‐TREM1‐treated diabetic wounds at day 4 show basal keratin marker K5, and neutrophil marker Ly6G, FOXM1, and citH3. Treatment of wounds with ⍺‐TREM1 resulted in increased FOXM1+ and decreased citH3+ neutrophils compared to vehicle‐treated diabetic wounds. (Scale bar: 50 μm). Quantification of mean fluorescence intensity was performed with Fiji software. n = 7 diabetic vehicle wounds and 6 diabetic ⍺‐TREM1 wounds. Data presented as mean ± SD. *P < 0.05, *P < 0.05, and **P < 0.01 (two‐tailed unpaired Student's t‐test).
- A
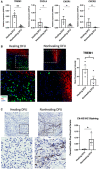
qPCR of TREM1 and genes involved in neutrophil recruitment demonstrate increased expression in healing DFUs compared to nonhealing DFUs (n = 5 healing and n = 6 nonhealing). Data presented as mean ± SD. *P < 0.05. **P < 0.01 (two‐tailed unpaired Student's t‐test).
- B
Representative images of healing and nonhealing DFUs show basal keratin marker K5 and TREM1 and corresponding quantification from healing (n = 4) and nonhealing (n = 4) is shown in the graph. Data presented as mean ± SEM. *P < 0.05 (two‐tailed unpaired Student's t‐test). (Scale bar: 50 μm).
- C
Representative images of cit‐H3 immunohistochemistry show increase staining in nonhealing DFUs when compared to healing DFUs, which was confirmed by corresponding quantification from healing (n = 3) and nonhealing (n = 3), shown in the graph. Data presented as mean ± SD. *P < 0.05 (two‐tailed unpaired Student's t‐test). (Scale bar: 50 μm).
References
-
- Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS (2014) Diabetic foot ulcers: Part I. pathophysiology and prevention. J Am Acad Dermatol 70: 1–18 - PubMed
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30: 459–489 - PubMed
-
- Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376: 2367–2375 - PubMed
-
- Bouchon A, Dietrich J, Colonna M (2000) Cutting edge: inflammatory responses can be triggered by TREM‐1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164: 4991–4995 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

