Defucosylated mouse‑dog chimeric anti‑HER2 monoclonal antibody exerts antitumor activities in mouse xenograft models of canine tumors
- PMID: 35856438
- PMCID: PMC9350980
- DOI: 10.3892/or.2022.8366
Defucosylated mouse‑dog chimeric anti‑HER2 monoclonal antibody exerts antitumor activities in mouse xenograft models of canine tumors
Abstract
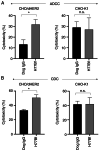
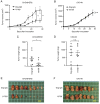
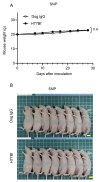
Human epidermal growth factor receptor 2 (HER2) overexpression has been reported in various types of cancer, including breast, gastric, lung, colorectal and pancreatic cancer. A humanized anti‑HER2 monoclonal antibody (mAb), trastuzumab, has been shown to improve survival of patients in HER2‑positive breast and gastric cancer. An anti‑HER2 mAb, H2Mab‑77 (mouse IgG1, kappa) was previously developed. In the present study, a defucosylated version of mouse‑dog chimeric anti‑HER2 mAb (H77Bf) was generated. H77Bf possesses a high binding‑affinity [a dissociation constant (KD): 7.5x10‑10 M, as determined by flow cytometric analysis] for dog HER2‑overexpressed CHO‑K1 (CHO/dHER2) cells. H77Bf highly exerted antibody‑dependent cellular cytotoxicity (ADCC) and complement‑dependent cytotoxicity (CDC) for CHO/dHER2 cells by canine mononuclear cells and complement, respectively. Moreover, administration of H77Bf significantly suppressed the development of CHO/dHER2 xenograft tumor in mice compared with the control dog IgG. H77Bf also possesses a high binding‑affinity (KD: 7.2x10‑10 M) for a canine mammary gland tumor cell line (SNP), and showed high ADCC and CDC activities for SNP cells. Intraperitoneal administration of H77Bf in mouse xenograft models of SNP significantly suppressed the development of SNP xenograft tumors compared with the control dog IgG. These results indicated that H77Bf exerts antitumor activities against dHER2‑positive canine cancers, and could be valuable treatment regimen for canine cancers.
Keywords: ADCC; CDC; HER2; antitumor activity; monoclonal antibody.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

