SARS-CoV-2 Convalescent Sera Binding and Neutralizing Antibody Concentrations Compared with COVID-19 Vaccine Efficacy Estimates against Symptomatic Infection
- PMID: 35856710
- PMCID: PMC9430572
- DOI: 10.1128/spectrum.01247-22
SARS-CoV-2 Convalescent Sera Binding and Neutralizing Antibody Concentrations Compared with COVID-19 Vaccine Efficacy Estimates against Symptomatic Infection
Abstract
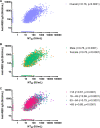
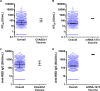
Previous COVID-19 vaccine efficacy (VE) studies have estimated neutralizing and binding antibody concentrations that correlate with protection from symptomatic infection; how these estimates compare to those generated in response to SARS-CoV-2 infection is unclear. Here, we assessed quantitative neutralizing and binding antibody concentrations using standardized SARS-CoV-2 assays on 3,067 serum specimens collected during 27 July 2020 to 27 August 2020 from COVID-19-unvaccinated persons with detectable anti-SARS-CoV-2 antibodies. Neutralizing and binding antibody concentrations were severalfold lower in the unvaccinated study population compared to published concentrations at 28 days postvaccination. In this convenience sample, ~88% of neutralizing and ~63 to 86% of binding antibody concentrations met or exceeded concentrations associated with 70% COVID-19 VE against symptomatic infection; ~30% of neutralizing and 1 to 14% of binding antibody concentrations met or exceeded concentrations associated with 90% COVID-19 VE. Our study not only supports observations of infection-induced immunity and current recommendations for vaccination postinfection to maximize protection against COVID-19, but also provides a large data set of pre-COVID-19 vaccination anti-SARS-CoV-2 antibody concentrations that will serve as an important comparator in the current setting of vaccine-induced and hybrid immunity. As new SARS-CoV-2 variants emerge and displace circulating virus strains, we recommend that standardized binding antibody assays that include spike protein-based antigens be utilized to estimate antibody concentrations correlated with protection from COVID-19. These estimates will be helpful in informing public health guidance, such as the need for additional COVID-19 vaccine booster doses to prevent symptomatic infection. IMPORTANCE Although COVID-19 vaccine efficacy (VE) studies have estimated antibody concentrations that correlate with protection from COVID-19, how these estimates compare to those generated in response to SARS-CoV-2 infection is unclear. We assessed quantitative neutralizing and binding antibody concentrations using standardized assays on serum specimens collected from COVID-19-unvaccinated persons with detectable antibodies. We found that most unvaccinated persons with qualitative antibody evidence of prior infection had quantitative antibody concentrations that met or exceeded concentrations associated with 70% VE against COVID-19. However, only a small proportion had antibody concentrations that met or exceeded concentrations associated with 90% VE, suggesting that persons with prior COVID-19 would benefit from vaccination to maximize protective antibody concentrations against COVID-19.
Keywords: COVID-19; IgG; SARS-CoV-2; anti-SARS-CoV-2; antibody; correlate of protection; correlation; immune; immunity; neutralizing antibodies; protection; quantitative; standardized.
Conflict of interest statement
The authors declare a conflict of interest. A.J.S., P.S.S., S.D., L.B., M.C., C.E., J.M.J., K.L.B., K.E.N.C., L.C.M., S.P., K.C., N.J.T., J.S., M.F., D.A., D.S., A.M.F., A.J.H., and A.V.G. have declared no potential conflicts of interest. K.C., M.B., M.F., C.J.P., T.W., S.C., D.A., D.S., and S.L. are/were employees of Labcorp during the study period. C.J.P. and S.L. have received grants or contracts on behalf of Labcorp-Monogram Biosciences from the United States Department of Health and Human Services/Biomedical Advanced Research and Development Authority and/or the United States Centers for Disease Control and Prevention. K.C. has a patent planned for a component of the Cov2Quant IgG Assay technology. K.C., M.B., C.J.P., T.W., S.C., D.S., and S.L. participate or have the option to participate in the Labcorp employee stock program.
Figures

References
-
- US Centers for Disease Control and Prevention. 2022. COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-.... Accessed 29 March 2022.
-
- Jones JM. 6 August 2021. About One in Five Americans Remain Vaccine-Resistant. https://news.gallup.com/poll/353081/one-five-americans-remain-vaccine-re....
-
- US Centers for Disease Control and Prevention. 2021. Science brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine.... Accessed 5 November 2021. - PubMed
-
- Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, Tostanoski LH, Yu J, Maliga Z, Nekorchuk M, Busman-Sahay K, Terry M, Wrijil LM, Ducat S, Martinez DR, Atyeo C, Fischinger S, Burke JS, Slein MD, Pessaint L, Van Ry A, Greenhouse J, Taylor T, Blade K, Cook A, Finneyfrock B, Brown R, Teow E, Velasco J, Zahn R, Wegmann F, Abbink P, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kordana N, Li Z, Lifton MA, Mahrokhian SH, Maxfield LF, Nityanandam R, Nkolola JP, Schmidt AG, Miller AD, Baric RS, Alter G, Sorger PK, Estes JD, Andersen H, Lewis MG, Barouch DH. 2020. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369:812–817. doi:10.1126/science.abc4776. - DOI - PMC - PubMed
-
- McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, Liu J, Peter L, Atyeo C, Zhu A, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Li Z, Nampanya F, Patel S, Pessaint L, Van Ry A, Blade K, Yalley-Ogunro J, Cabus M, Brown R, Cook A, Teow E, Andersen H, Lewis MG, Lauffenburger DA, Alter G, Barouch DH. 2021. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590:630–634. doi:10.1038/s41586-020-03041-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

