Nitrogenase Chemistry at 10 Kelvin─Phototautomerization and Recombination of CO-Inhibited α-H195Q Enzyme
- PMID: 35856737
- PMCID: PMC12037267
- DOI: 10.1021/acs.inorgchem.2c00818
Nitrogenase Chemistry at 10 Kelvin─Phototautomerization and Recombination of CO-Inhibited α-H195Q Enzyme
Abstract
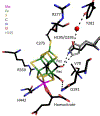
CO-bound forms of nitrogenase are N2-reduction inhibited and likely intermediates in Fischer-Tropsch chemistry. Visible-light photolysis at 7 K was used to interrogate all three known CO-related EPR-active forms as exhibited by the α-H195Q variant of Azotobacter vinelandii nitrogenase MoFe protein. The hi(5)-CO EPR signal converted to the hi-CO EPR signal, which reverted at 10 K. FT-IR monitoring revealed an exquisitely light-sensitive "Hi-2" species with bands at 1932 and 1866 cm-1 that yielded "Hi-1" with bands at 1969 and 1692 cm-1, which reverted to "Hi-2". The similarities of photochemical behavior and recombination kinetics showed, for the first time, that hi-CO EPR and "Hi-1" IR signals arise from one chemical species. hi(5)-CO EPR and "Hi-2" IR signals are from a second species, and lo-CO EPR and "Lo-2" IR signals, formed after prolonged illumination, are from a third species. Comparing FT-IR data with CO-inhibited MoFe-protein crystal structures allowed assignment of CO-bonding geometries in these species.
Figures


References
-
- Leigh GJ The World’s Greatest Fix -- A History of Nitrogen and Agriculture; Oxford University Press: New York, 2004.
-
- “The Haber-Bosch Process - Exemplar of 20th Century Chemical Industry”, Travis, T. Chem. Ind, 1993, 581–585.
-
- 1918 Nobel Lecture in Chemistry, Haber F., Ed.; Elsevier: Amsterdam, 1966.
-
- “How a century of ammonia synthesis changed the world”, Erisman JW.; Sutton MA.; Galloway J.; Klimont Z.; Winiwarter W. Nature Geoscience, 2008, 1, 636–639.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

