Gender-based effect of absence of gut microbiota on the protective efficacy of Bifidobacterium longum-fermented rice bran diet against inflammation-associated colon tumorigenesis
- PMID: 35856887
- PMCID: PMC9474629
- DOI: 10.1002/mc.23452
Gender-based effect of absence of gut microbiota on the protective efficacy of Bifidobacterium longum-fermented rice bran diet against inflammation-associated colon tumorigenesis
Abstract
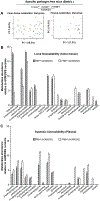
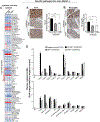
Dietary rice bran (RB) has shown capacity to influence metabolism by modulation of gut microbiota in individuals at risk for colorectal cancer (CRC), which warranted attention for delineating mechanisms for bidirectional influences and cross-feeding between the host and RB-modified gut microbiota to reduce CRC. Accordingly, in the present study, fermented rice bran (FRB, fermented with a RB responsive microbe Bifidobacterium longum), and non-fermented RB were fed as 10% w/w (diet) to gut microbiota-intactspf or germ-free micegf to investigate comparative efficacy against inflammation-associated azoxymethane/dextran sodium sulfate (AOM/DSS)-induced CRC. Results indicated both microbiota-dependent and independent mechanisms for RB meditated protective efficacy against CRC that was associated with reduced neoplastic lesion size and local-mucosal/systemic inflammation, and restoration of colonic epithelial integrity. Enrichment of beneficial commensals (such as, Clostridiales, Blautia, Roseburia), phenolic metabolites (benzoate and catechol metabolism), and dietary components (ferulic acid-4 sulfate, trigonelline, and salicylate) were correlated with anti-CRC efficacy. Germ-free studies revealed gender-specific physiological variables could differentially impact CRC growth and progression. In the germ-free females, the RB dietary treatment showed a ∼72% reduction in the incidence of colonic epithelial erosion when compared to the ∼40% reduction in FRB-fed micegf . Ex vivo fermentation of RB did not parallel the localized-protective benefits of gut microbial metabolism by RB in damaged colonic tissues. Findings from this study suggest potential needs for safety considerations of fermented fiber rich foods as dietary strategies against severe inflammation-associated colon tumorigenesis (particularly with severe damage to the colonic epithelium).
Keywords: azoxymethane; cancer intervention; colon carcinogenesis; fermentation; germ free mice; metabolomics; microbiome; probiotics; rice bran.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Figures





Similar articles
-
Bifidobacterium longum-fermented rice bran and rice bran supplementation affects the gut microbiome and metabolome.Benef Microbes. 2019 Dec 9;10(8):823-839. doi: 10.3920/BM2019.0017. Epub 2019 Sep 29. Benef Microbes. 2019. PMID: 31965839 Free PMC article.
-
Role of Dietary Defatted Rice Bran in the Modulation of Gut Microbiota in AOM/DSS-Induced Colitis-Associated Colorectal Cancer Rat Model.Nutrients. 2023 Mar 22;15(6):1528. doi: 10.3390/nu15061528. Nutrients. 2023. PMID: 36986258 Free PMC article.
-
Dietary Rice Bran-Modified Human Gut Microbial Consortia Confers Protection against Colon Carcinogenesis Following Fecal Transfaunation.Biomedicines. 2021 Feb 3;9(2):144. doi: 10.3390/biomedicines9020144. Biomedicines. 2021. PMID: 33546192 Free PMC article.
-
Production and Utilization of Fermented Rice Bran as Animal Feed.Anim Sci J. 2025 Jan-Dec;96(1):e70037. doi: 10.1111/asj.70037. Anim Sci J. 2025. PMID: 39947126 Free PMC article. Review.
-
Exploring the Role of Gut Microbiome in Colon Cancer.Appl Biochem Biotechnol. 2021 Jun;193(6):1780-1799. doi: 10.1007/s12010-021-03498-9. Epub 2021 Jan 25. Appl Biochem Biotechnol. 2021. PMID: 33492552 Review.
Cited by
-
OncoSexome: the landscape of sex-based differences in oncologic diseases.Nucleic Acids Res. 2025 Jan 6;53(D1):D1443-D1459. doi: 10.1093/nar/gkae1003. Nucleic Acids Res. 2025. PMID: 39535034 Free PMC article.
-
Unveiling the Therapeutic Potential of Trigonelline: A Promising Approach in Cancer Prevention and Treatment.Anticancer Agents Med Chem. 2025;25(16):1175-1187. doi: 10.2174/0118715206363456250226061713. Anticancer Agents Med Chem. 2025. PMID: 40045859 Review.
-
Rice Bran Extraction and Stabilization Methods for Nutrient and Phytochemical Biofortification, Nutraceutical Development, and Dietary Supplementation.Nutr Rev. 2025 Apr 1;83(4):692-712. doi: 10.1093/nutrit/nuae174. Nutr Rev. 2025. PMID: 39657228 Free PMC article. Review.
-
Sexual Dimorphism in Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet.Nutrients. 2023 May 2;15(9):2175. doi: 10.3390/nu15092175. Nutrients. 2023. PMID: 37432375 Free PMC article.
-
Anti-Colorectal Cancer Effects of Inonotus hispidus (Bull.: Fr.) P. Karst. Spore Powder through Regulation of Gut Microbiota-Mediated JAK/STAT Signaling.Nutrients. 2022 Aug 12;14(16):3299. doi: 10.3390/nu14163299. Nutrients. 2022. PMID: 36014805 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

