Expert opinion on NSCLC small specimen biomarker testing - Part 2: Analysis, reporting, and quality assessment
- PMID: 35857103
- PMCID: PMC9297263
- DOI: 10.1007/s00428-022-03344-1
Expert opinion on NSCLC small specimen biomarker testing - Part 2: Analysis, reporting, and quality assessment
Abstract
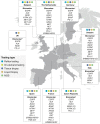
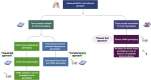
The diagnostic work-up for non-small cell lung cancer (NSCLC) requires biomarker testing to guide therapy choices. This article is the second of a two-part series. In Part 1, we summarised evidence-based recommendations for obtaining and processing small specimen samples (i.e. pre-analytical steps) from patients with advanced NSCLC. Here, in Part 2, we summarise evidence-based recommendations relating to analytical steps of biomarker testing (and associated reporting and quality assessment) of small specimen samples in NSCLC. As the number of biomarkers for actionable (genetic) targets and approved targeted therapies continues to increase, simultaneous testing of multiple actionable oncogenic drivers using next-generation sequencing (NGS) becomes imperative, as set forth in European Society for Medical Oncology guidelines. This is particularly relevant in advanced NSCLC, where tissue specimens are typically limited and NGS may help avoid tissue exhaustion compared with sequential biomarker testing. Despite guideline recommendations, significant discrepancies in access to NGS persist across Europe, primarily due to reimbursement constraints. The use of increasingly complex testing methods also has implications for the reporting of results. Molecular testing reports should include clinical interpretation with additional commentary on sample adequacy as appropriate. Molecular tumour boards are recommended to facilitate the interpretation of complex genetic information arising from NGS, and to collaboratively determine the optimal treatment for patients with NSCLC. Finally, whichever testing modality is employed, it is essential that adequate internal and external validation and quality control measures are implemented.
Keywords: Best practice; External quality assessment; Liquid biopsy; Molecular diagnostics; Next-generation sequencing; Non-small cell lung carcinoma.
© 2022. The Author(s).
Conflict of interest statement
FP-L has provided consultancy for AbbVie, Amgen, AstraZeneca, Bayer, BMS, Clovis, Daiichi Sankyo, Diaceutics, Eli Lilly, Illumina, Invitae, MSD, Novartis, Pfizer, Roche, and Ventana, and has received research grants from AbbVie, AstraZeneca, Bayer, BMS, Illumina, MSD, and Roche. KMK has provided consultancy for AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Diaceutics, Eli Lilly, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Ventana. PG has provided consultancy for AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. She has been a speaker for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. ET has received honoraria from Amgen, Bayer, BMS, MSD, Pfizer, Roche, and Takeda, and grants to VU Medical Center from AbbVie and Pfizer. ED has received grants from Amgen, AstraZeneca, and Pfizer. NN has received speaker’s fees from and/or participated in advisory boards for AstraZeneca, Bayer, Biocartis, BMS, Eli Lilly, Illumina, Incyte, Novartis, Merck, MSD, Qiagen, Roche, Sanofi, and Thermo Fisher; and financial support for research projects from AstraZeneca, Biocartis, Blueprint, Illumina, Merck, QIAGEN, Roche, and Thermo Fisher. SJP has received honoraria from AstraZeneca, and grants from Amgen, AstraZeneca, and Merck. JF has provided consultancy for Eli Lilly. JK is employed by Amgen and has stocks/shares in Amgen. DdR is employed by Amgen. AR has received honoraria from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, MSD, Novartis, Pfizer, and Roche, and grants from AstraZeneca and Pfizer. HM has provided consultancy for AstraZeneca, Bayer, BMS, Diaceutics, and Roche, and has received research grants from Roche.
Figures

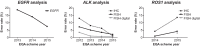
References
-
- European Society for Medical Oncology (ESMO) (2020) Metastatic non-small-cell lung cancer: ESMO cinical practice guidelines for diagnosis, treatment and follow-up. Available from: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-1.... (cited 2021 25 November)
-
- Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr K, Kwiatkowski DJ, Ladanyi M, Nowak JA, Sholl L, Temple-Smolkin R, Solomon B, Souter LH, Thunnissen E, Tsao MS, Ventura CB, Wynes MW, Yatabe Y. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20:129–159. doi: 10.1016/j.jmoldx.2017.11.004. - DOI - PubMed
-
- Lester J, Escriu C, Khan S, Hudson E, Mansy T, Conn A, Chan S, Powell C, Brock J, Conibear J, Nelless L, Nayar V, Zhuo X, Durand A, Amin A, Martin P, Zhang X, Pawar V. Retrospective analysis of real-world treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer starting first-line systemic therapy in the United Kingdom. BMC Cancer. 2021;21:515. doi: 10.1186/s12885-021-08096-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

