Immunogenic properties of SARS-CoV-2 inactivated by ultraviolet light
- PMID: 35857146
- PMCID: PMC9296761
- DOI: 10.1007/s00705-022-05530-7
Immunogenic properties of SARS-CoV-2 inactivated by ultraviolet light
Abstract
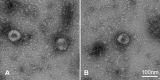
Vaccination against COVID-19 is the most effective method of controlling the spread of SARS-CoV-2 and reducing mortality from this disease. The development of vaccines with high protective activity against a wide range of SARS-CoV-2 antigenic variants remains relevant. In this regard, evaluation of the effectiveness of physical methods of virus inactivation, such as ultraviolet irradiation (UV) of the virus stock, remains relevant. This study demonstrates that the UV treatment of SARS-CoV-2 completely inactivates its infectivity while preserving its morphology, antigenic properties, and ability to induce the production of virus-neutralizing antibodies in mice through immunization. Thus, the UV inactivation of SARS-CoV-2 makes it possible to obtain viral material similar in its antigenic and immunogenic properties to the native antigen, which can be used both for the development of diagnostic test systems and for the development of an inactivated vaccine against COVID-19.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
A human cell-based SARS-CoV-2 vaccine elicits potent neutralizing antibody responses and protects mice from SARS-CoV-2 challenge.Emerg Microbes Infect. 2021 Dec;10(1):1555-1573. doi: 10.1080/22221751.2021.1957400. Emerg Microbes Infect. 2021. PMID: 34304724 Free PMC article.
-
Single Immunization with Recombinant ACAM2000 Vaccinia Viruses Expressing the Spike and the Nucleocapsid Proteins Protects Hamsters against SARS-CoV-2-Caused Clinical Disease.J Virol. 2022 May 11;96(9):e0038922. doi: 10.1128/jvi.00389-22. Epub 2022 Apr 12. J Virol. 2022. PMID: 35412347 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Analysis of the Protective Efficacy of Approved COVID-19 Vaccines Against Various Mutants.Front Immunol. 2022 Apr 28;13:804945. doi: 10.3389/fimmu.2022.804945. eCollection 2022. Front Immunol. 2022. PMID: 35572594 Free PMC article. Review.
-
SARS-CoV-2: Emergence of New Variants and Effectiveness of Vaccines.Front Cell Infect Microbiol. 2021 Dec 14;11:777212. doi: 10.3389/fcimb.2021.777212. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34970509 Free PMC article. Review.
Cited by
-
Insights into the Pathogenesis and Development of Recombinant Japanese Encephalitis Virus Genotype 3 as a Vaccine.Vaccines (Basel). 2024 May 30;12(6):597. doi: 10.3390/vaccines12060597. Vaccines (Basel). 2024. PMID: 38932326 Free PMC article.
-
Cold-adapted SARS-CoV-2 variants with different temperature sensitivity exhibit an attenuated phenotype and confer protective immunity.Vaccine. 2023 Jan 23;41(4):892-902. doi: 10.1016/j.vaccine.2022.12.019. Epub 2022 Dec 13. Vaccine. 2023. PMID: 36528447 Free PMC article.
-
SARS-CoV-2 Modulation of HIV Latency Reversal in a Myeloid Cell Line: Direct and Bystander Effects.Viruses. 2024 Aug 17;16(8):1310. doi: 10.3390/v16081310. Viruses. 2024. PMID: 39205284 Free PMC article.
-
SARS-CoV-2 Impairs Osteoblast Differentiation Through Spike Glycoprotein and Cytokine Dysregulation.Viruses. 2025 Jan 22;17(2):143. doi: 10.3390/v17020143. Viruses. 2025. PMID: 40006897 Free PMC article.
-
Exposure of monocyte-derived macrophages to the UV-inactivated SARS-CoV-2 VOCs shows similar effects on the transcriptomic profile as active virus: a comparative analysis.J Transl Med. 2025 Mar 14;23(1):332. doi: 10.1186/s12967-025-06264-1. J Transl Med. 2025. PMID: 40087733 Free PMC article. No abstract available.
References
-
- Kozlovskaya LI, Piniaeva AN, Ignatyev GM, Gordeychuk IV, Volok VP, Rogova YV, Shishova AA, Kovpak AA, Ivin YY, Antonova LP, Mefyod KM, Prokosheva LS, Sibirkina AS, Tarasova YY, Bayurova EO, Gancharova OS, Illarionova VV, Glukhov GS, Sokolova OS, Shaitan KV, Moysenovich AM, Gulyaev SA, Gulyaeva TV, Moroz AV, Gmyl LV, Ipatova EG, Kirpichnikov MP, Egorov AM, Siniugina AA, Ishmukhametov AA. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerg Microbes Infect. 2021 doi: 10.1080/22221751.2021.1971569. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

