Cannabinoid Content and Label Accuracy of Hemp-Derived Topical Products Available Online and at National Retail Stores
- PMID: 35857320
- PMCID: PMC9301515
- DOI: 10.1001/jamanetworkopen.2022.23019
Cannabinoid Content and Label Accuracy of Hemp-Derived Topical Products Available Online and at National Retail Stores
Abstract
Importance: Products containing cannabinoids such as cannabidiol (CBD) have proliferated since 2018, when the Agriculture Improvement Act removed hemp (ie, cannabis containing <0.3% Δ9-tetrahydrocannabinol [THC]) from the US controlled substances list. Topical cannabinoid products can be purchased nationwide at retail stores and over the internet, yet research on these products is scarce.
Objective: To evaluate the cannabinoid content (ie, CBD and THC) and label accuracy of topical cannabinoid products and to quantify their therapeutic and nontherapeutic claims.
Design, setting, and participants: Product inclusion criteria included designation as hemp products, intended for topical or transdermal application, and purported to contain cannabinoids (eg, CBD). All unique products available at each retail store were purchased. Online products were identified via Google using relevant keywords (eg, hemp or CBD topical). Various products (eg, lotions and patches) were purchased from retail stores (eg, pharmacies, grocery stores, and cosmetic or beauty stores) in Baltimore, Maryland, and online. Data analysis was performed from March to June 2022.
Main outcomes and measures: Labeled and actual total amounts of CBD and THC, measured via gas chromatography-mass spectrometry. Therapeutic and nontherapeutic claims and references to the US Food and Drug Administration were quantified.
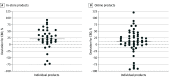
Results: A total of 105 products were purchased, 45 from retail locations and 60 online. Of the 89 products that listed a total amount of CBD on the label, 18% (16 products) were overlabeled (ie, contained >10% less CBD than advertised), 58% (52 products) were underlabeled (ie, contained >10% more CBD than advertised), and 24% (21 products) were accurately labeled. The median (range) percentage deviation between the actual total amount of CBD and the labeled amount was 21% (-75% to 93%) for in-store products and 10% (-96% to 121%) for online products, indicating that products contained more CBD than advertised overall. THC was detected in 37 of 105 products (35%), although all contained less than 0.3% THC. Among the 37 THC-containing products, 4 (11%) were labeled as THC free, 14 (38%) indicated they contained less than 0.3% THC, and 19 (51%) did not reference THC on the label. Overall, 28% of products (29 products) made therapeutic claims, 14% (15 products) made cosmetic claims, and only 47% (49 products) noted that they were not Food and Drug Administration approved.
Conclusions and relevance: In a case series of topical cannabinoid products purchased online and at popular retail stores, products were often inaccurately labeled for CBD and many contained THC. These findings suggest that clinical studies are needed to determine whether topical cannabinoid products with THC can produce psychoactive effects or positive drug tests for cannabis.
Conflict of interest statement
Figures
Similar articles
-
Cannabinoid Content and Label Accuracy of Various Hemp-Derived Haircare, Cosmetic, and Edible Products Available at Retail Stores and Online in the United States.Cannabis Cannabinoid Res. 2024 Jul 19. doi: 10.1089/can.2024.0039. Online ahead of print. Cannabis Cannabinoid Res. 2024. PMID: 39029473
-
Analysis of cannabidiol (CBD) and THC in nonprescription consumer products: Implications for patients and practitioners.Epilepsy Behav. 2022 Feb;127:108514. doi: 10.1016/j.yebeh.2021.108514. Epub 2022 Jan 5. Epilepsy Behav. 2022. PMID: 34998268
-
Pharmacokinetics and pharmacodynamics of five distinct commercially available hemp-derived topical cannabidiol products.J Anal Toxicol. 2024 Mar 1;48(2):81-98. doi: 10.1093/jat/bkae001. J Anal Toxicol. 2024. PMID: 38217086
-
Using the BMD Approach to Derive Acceptable Daily Intakes of Cannabidiol (CBD) and Tetrahydrocannabinol (THC) Relevant to Electronic Cigarette Liquids.Front Biosci (Landmark Ed). 2022 Jul 25;27(8):228. doi: 10.31083/j.fbl2708228. Front Biosci (Landmark Ed). 2022. PMID: 36042166
-
The Impact of Cannabidiol on Psychiatric and Medical Conditions.J Clin Med Res. 2020 Jul;12(7):393-403. doi: 10.14740/jocmr4159. Epub 2020 Jun 25. J Clin Med Res. 2020. PMID: 32655732 Free PMC article. Review.
Cited by
-
Safety and risks of CBD oils purchased online: unveiling uncertain quality and vague health claims.Front Pharmacol. 2023 Dec 14;14:1273540. doi: 10.3389/fphar.2023.1273540. eCollection 2023. Front Pharmacol. 2023. PMID: 38192407 Free PMC article.
-
Comprehensive Insight into Cutaneous Application of Hemp.Pharmaceutics. 2024 May 31;16(6):748. doi: 10.3390/pharmaceutics16060748. Pharmaceutics. 2024. PMID: 38931870 Free PMC article. Review.
-
Prevalence of cannabidiol use and correlates in U.S. adults.Drug Alcohol Depend Rep. 2024 Oct 9;13:100289. doi: 10.1016/j.dadr.2024.100289. eCollection 2024 Dec. Drug Alcohol Depend Rep. 2024. PMID: 39831101 Free PMC article.
-
Self-reported adverse events associated with ∆8-Tetrahydrocannabinol (Delta-8-THC) Use.J Cannabis Res. 2023 May 23;5(1):15. doi: 10.1186/s42238-023-00191-y. J Cannabis Res. 2023. PMID: 37217977 Free PMC article.
-
Dispensary Cannabidiol (CBD): Nothing to Worry About!Child Neurol Open. 2023 Apr 19;10:2329048X231169395. doi: 10.1177/2329048X231169395. eCollection 2023 Jan-Dec. Child Neurol Open. 2023. PMID: 37101430 Free PMC article.
References
-
- Bridges M, Hanson K. Regulating hemp and cannabis-based products. NCSL Legisbrief. 2017;25(37):1-2. - PubMed

