Rapid liquid phase-assisted ultrahigh-temperature sintering of high-entropy ceramic composites
- PMID: 35857462
- PMCID: PMC9258815
- DOI: 10.1126/sciadv.abn8241
Rapid liquid phase-assisted ultrahigh-temperature sintering of high-entropy ceramic composites
Abstract
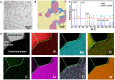
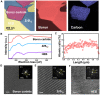
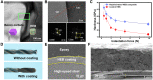
High-entropy ceramics and their composites display high mechanical strength and attractive high-temperature stabilities. However, properties like strong covalent bond character and low self-diffusion coefficients make them difficult to get sintered, limiting their mass popularity. Here, we present a rapid liquid phase-assisted ultrahigh-temperature sintering strategy and use high-entropy metal diboride/boron carbide composite as a proof of concept. We use a carbon-based heater to fast-heat the composite to around 3000 K, and a small fraction of eutectic liquid was formed at the interface between high-entropy metal diborides and boron carbide. A crystalline dodecaboride intergranular phase was generated upon cooling to ameliorate the adhesion between the components. The as-sintered composite presents a high hardness of 36.4 GPa at a load of 0.49 N and 24.4 GPa at a load of 9.8 N. This liquid phase-assisted rapid ultrahigh-temperature strategy can be widely applicable for other ultrahigh-temperature ceramics as well.
Figures


References
-
- Qin M., Gild J., Wang H., Harrington T., Vecchio K. S., Luo J., Dissolving and stabilizing soft WB2 and MoB2 phases into high-entropy borides via boron-metals reactive sintering to attain higher hardness. J. Eur. Ceram. Soc. 40, 4348–4353 (2020).
-
- Gild J., Kaufmann K., Vecchio K., Luo J., Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scr. Mater. 170, 106–110 (2019).
-
- Braic V., Vladescu A., Balaceanu M., Luculescu C. R., Braic M., Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coatings Technol. 211, 117–121 (2012).
-
- Feng L., Fahrenholtz W. G., Hilmas G. E., Zhou Y., Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 162, 90–93 (2019).
LinkOut - more resources
Full Text Sources
Other Literature Sources

