Transplanted organoids empower human preclinical assessment of drug candidate for the clinic
- PMID: 35857479
- PMCID: PMC9258952
- DOI: 10.1126/sciadv.abj5633
Transplanted organoids empower human preclinical assessment of drug candidate for the clinic
Abstract
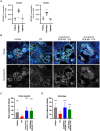
Pharmacodynamic (PD) studies are an essential component of preclinical drug discovery. Current approaches for PD studies, including the analysis of novel kidney disease targeting therapeutic agents, are limited to animal models with unclear translatability to the human condition. To address this challenge, we developed a novel approach for PD studies using transplanted, perfused human kidney organoids. We performed pharmacokinetic (PK) studies with GFB-887, an investigational new drug now in phase 2 trials. Orally dosed GFB-887 to athymic rats that had undergone organoid transplantation resulted in measurable drug exposure in transplanted organoids. We established the efficacy of orally dosed GFB-887 in PD studies, where quantitative analysis showed significant protection of kidney filter cells in human organoids and endogenous rat host kidneys. This widely applicable approach demonstrates feasibility of using transplanted human organoids in preclinical PD studies with an investigational new drug, empowering organoids to revolutionize drug discovery.
Figures


References
-
- Inrig J. K., Califf R. M., Tasneem A., Vegunta R. K., Molina C., Stanifer J. W., Chiswell K., Patel U. D., The landscape of clinical trials in nephrology: A systematic review of Clinicaltrials.gov. Am. J. Kidney Dis. 63, 771–780 (2014). - PMC - PubMed
-
- Jager K. J., Kovesdy C., Langham R., Rosenberg M., Jha V., Zoccali C., A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 96, 1048–1050 (2019). - PubMed
-
- Takahashi T., Organoids for drug discovery and personalized medicine. Annu. Rev. Pharmacol. Toxicol. 59, 447–462 (2019). - PubMed
-
- Dvela-Levitt M., Kost-Alimova M., Emani M., Kohnert E., Thompson R., Sidhom E. H., Rivadeneira A., Sahakian N., Roignot J., Papagregoriou G., Montesinos M. S., Clark A. R., McKinney D., Gutierrez J., Roth M., Ronco L., Elonga E., Carter T. A., Gnirke A., Melanson M., Hartland K., Wieder N., Hsu J. C., Deltas C., Hughey R., Bleyer A. J., Kmoch S., Zivna M., Baresova V., Kota S., Schlondorff J., Heiman M., Alper S. L., Wagner F., Weins A., Golub T. R., Lander E. S., Greka A., Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell 178, 521–535.e23 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

