Tracking the immune response by MRI using biodegradable and ultrasensitive microprobes
- PMID: 35857494
- PMCID: PMC9278862
- DOI: 10.1126/sciadv.abm3596
Tracking the immune response by MRI using biodegradable and ultrasensitive microprobes
Abstract
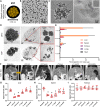
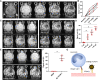
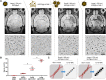
Molecular magnetic resonance imaging (MRI) holds great promise for diagnosis and therapeutic monitoring in a wide range of diseases. However, the low intrinsic sensitivity of MRI to detect exogenous contrast agents and the lack of biodegradable microprobes have prevented its clinical development. Here, we synthetized a contrast agent for molecular MRI based on a previously unknown mechanism of self-assembly of catechol-coated magnetite nanocrystals into microsized matrix-based particles. The resulting biodegradable microprobes (M3P for microsized matrix-based magnetic particles) carry up to 40,000 times higher amounts of superparamagnetic material than classically used nanoparticles while preserving favorable biocompatibility and excellent water dispersibility. After conjugation to monoclonal antibodies, targeted M3P display high sensitivity and specificity to detect inflammation in vivo in the brain, kidneys, and intestinal mucosa. The high payload of superparamagnetic material, excellent toxicity profile, short circulation half-life, and widespread reactivity of the M3P particles provides a promising platform for clinical translation of immuno-MRI.
Figures



References
-
- Lohrke J., Frenzel T., Endrikat J., Alves F. C., Grist T. M., Law M., Lee J. M., Leiner T., Li K.-C., Nikolaou K., Prince M. R., Schild H. H., Weinreb J. C., Yoshikawa K., Pietsch H., 25 years of contrast-enhanced MRI: Developments, current challenges and future perspectives. Adv. Ther. 33, 1–28 (2016). - PMC - PubMed
-
- Caravan P., Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 35, 512–523 (2006). - PubMed
-
- James M. L., Gambhir S. S., A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012). - PubMed
-
- Laurent S., Forge D., Port M., Roch A., Robic C., Vander Elst L., Muller R. N., Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008). - PubMed
LinkOut - more resources
Full Text Sources

