Overexpression of the scopoletin biosynthetic pathway enhances lignocellulosic biomass processing
- PMID: 35857515
- PMCID: PMC9278857
- DOI: 10.1126/sciadv.abo5738
Overexpression of the scopoletin biosynthetic pathway enhances lignocellulosic biomass processing
Abstract
Lignin is the main factor limiting the enzymatic conversion of lignocellulosic biomass into fermentable sugars. To reduce the recalcitrance engendered by the lignin polymer, the coumarin scopoletin was incorporated into the lignin polymer through the simultaneous expression of FERULOYL-CoA 6'-HYDROXYLASE 1 (F6'H1) and COUMARIN SYNTHASE (COSY) in lignifying cells in Arabidopsis. The transgenic lines overproduced scopoletin and incorporated it into the lignin polymer, without adversely affecting plant growth. About 3.3% of the lignin units in the transgenic lines were derived from scopoletin, thereby exceeding the levels of the traditional p-hydroxyphenyl units. Saccharification efficiency of alkali-pretreated scopoletin-overproducing lines was 40% higher than for wild type.
Figures
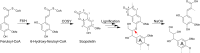


Similar articles
-
Effects of feruloyl-CoA 6'-hydroxylase 1 overexpression on lignin and cell wall characteristics in transgenic hybrid aspen.Front Plant Sci. 2025 Mar 28;16:1543168. doi: 10.3389/fpls.2025.1543168. eCollection 2025. Front Plant Sci. 2025. PMID: 40225026 Free PMC article.
-
Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana.Plant J. 2008 Sep;55(6):989-99. doi: 10.1111/j.1365-313X.2008.03568.x. Epub 2008 Jun 10. Plant J. 2008. PMID: 18547395
-
Introducing curcumin biosynthesis in Arabidopsis enhances lignocellulosic biomass processing.Nat Plants. 2019 Feb;5(2):225-237. doi: 10.1038/s41477-018-0350-3. Epub 2019 Jan 28. Nat Plants. 2019. PMID: 30692678
-
Glycerol organosolv pretreatment can unlock lignocellulosic biomass for production of fermentable sugars: Present situation and challenges.Bioresour Technol. 2022 Jan;344(Pt B):126264. doi: 10.1016/j.biortech.2021.126264. Epub 2021 Nov 2. Bioresour Technol. 2022. PMID: 34737053 Review.
-
Biomass recalcitrance: a multi-scale, multi-factor, and conversion-specific property.J Exp Bot. 2015 Jul;66(14):4109-18. doi: 10.1093/jxb/erv267. Epub 2015 Jun 9. J Exp Bot. 2015. PMID: 26060266 Review.
Cited by
-
Enhancing monolignol ferulate conjugate levels in poplar lignin via OsFMT1.Biotechnol Biofuels Bioprod. 2024 Jul 13;17(1):97. doi: 10.1186/s13068-024-02544-y. Biotechnol Biofuels Bioprod. 2024. PMID: 39003470 Free PMC article.
-
Biotechnological Potential of the Stress Response and Plant Cell Death Regulators Proteins in the Biofuel Industry.Cells. 2023 Aug 8;12(16):2018. doi: 10.3390/cells12162018. Cells. 2023. PMID: 37626829 Free PMC article.
-
Rapidly mining candidate cotton drought resistance genes based on key indicators of drought resistance.BMC Plant Biol. 2024 Feb 21;24(1):129. doi: 10.1186/s12870-024-04801-6. BMC Plant Biol. 2024. PMID: 38383284 Free PMC article.
-
Breeding for improved digestibility and processing of lignocellulosic biomass in Zea mays.Front Plant Sci. 2024 Jul 26;15:1419796. doi: 10.3389/fpls.2024.1419796. eCollection 2024. Front Plant Sci. 2024. PMID: 39129761 Free PMC article. Review.
-
Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo.Int J Mol Sci. 2025 Apr 27;26(9):4158. doi: 10.3390/ijms26094158. Int J Mol Sci. 2025. PMID: 40362397 Free PMC article.
References
-
- Nicholson R. L., Hammerschmidt R., Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 30, 369–389 (1992).
-
- Vanholme R., De Meester B., Ralph J., Boerjan W., Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 56, 230–239 (2019). - PubMed
-
- Del Río J. C., Marques G., Rencoret J., Martínez Á. T., Gutiérrez A., Occurrence of naturally acetylated lignin units. J. Agric. Food Chem. 55, 5461–5468 (2007). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

