Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery
- PMID: 35857516
- PMCID: PMC9286503
- DOI: 10.1126/sciadv.abo6163
Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery
Abstract
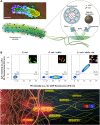
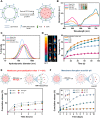
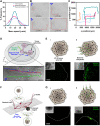
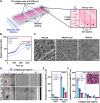
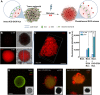
Bacterial biohybrids, composed of self-propelling bacteria carrying micro/nanoscale materials, can deliver their payload to specific regions under magnetic control, enabling additional frontiers in minimally invasive medicine. However, current bacterial biohybrid designs lack high-throughput and facile construction with favorable cargoes, thus underperforming in terms of propulsion, payload efficiency, tissue penetration, and spatiotemporal operation. Here, we report magnetically controlled bacterial biohybrids for targeted localization and multistimuli-responsive drug release in three-dimensional (3D) biological matrices. Magnetic nanoparticles and nanoliposomes loaded with photothermal agents and chemotherapeutic molecules were integrated onto Escherichia coli with ~90% efficiency. Bacterial biohybrids, outperforming previously reported E. coli-based microrobots, retained their original motility and were able to navigate through biological matrices and colonize tumor spheroids under magnetic fields for on-demand release of the drug molecules by near-infrared stimulus. Our work thus provides a multifunctional microrobotic platform for guided locomotion in 3D biological networks and stimuli-responsive delivery of therapeutics for diverse medical applications.
Figures
References
-
- M. Sitti, Mobile Microrobotics (MIT Press, 2017).
-
- Erkoc P., Yasa I. C., Ceylan H., Yasa O., Alapan Y., Sitti M., Mobile microrobots for active therapeutic delivery. Adv. Ther. 2, 1800064 (2019).
-
- Alapan Y., Bozuyuk U., Erkoc P., Karacakol A. C., Sitti M., Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow. Sci. Robot. 5, eaba5726 (2020). - PubMed
-
- Alapan Y., Yigit B., Beker O., Demirörs A. F., Sitti M., Shape-encoded dynamic assembly of mobile micromachines. Nat. Mater. 18, 1244–1251 (2019). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

