Changing the Antipsychotic in Early Nonimprovers to Amisulpride or Olanzapine: Randomized, Double-Blind Trial in Patients With Schizophrenia
- PMID: 35857811
- PMCID: PMC9673269
- DOI: 10.1093/schbul/sbac068
Changing the Antipsychotic in Early Nonimprovers to Amisulpride or Olanzapine: Randomized, Double-Blind Trial in Patients With Schizophrenia
Abstract
Background and hypothesis: Meta-analyses have shown that the majority of patients with schizophrenia who have not improved after 2 weeks of treatment with an antipsychotic drug are unlikely to fully respond later. We hypothesized that switching to another antipsychotic with a different receptor binding profile is an effective strategy in such a situation.
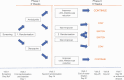
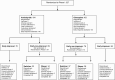
Study design: In total, 327 inpatients with an acute exacerbation of schizophrenia were randomized to double-blind treatment with either olanzapine (5-20 mg/day) or amisulpride (200-800 mg/day). Those patients who had not reached at least 25% Positive-and-Negative-Syndrome-Scale (PANSS) total score reduction from baseline after 2 weeks (the "non-improvers") were rerandomized double-blind to either staying on the same compound ("stayers") or to switching to the other antipsychotic ("switchers") for another 6 weeks. The primary outcome was the difference in the number of patients in symptomatic remission between the combined "switchers" and the "stayers" after 8 weeks of treatment, analyzed by logistic regression.
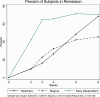
Study results: A total of 142 nonimprovers were rerandomized at week two. 25 (45.5 %) of the 'stayers' compared to 41 (68.3 %) of the "switchers" reached remission at endpoint (p = .006). Differences in secondary efficacy outcomes were not significant, except for the PANSS negative subscore and the Clinical-Global-Impression-Scale. "Switchers" and "stayers" did not differ in safety outcomes.
Conclusions: Switching "non-improvers" from amisulpride to olanzapine or vice-versa increased remission rates and was safe. The superiority in the primary outcome was, however, not paralleled by significant differences in most secondary efficacy outcomes and the effect was only apparent at the last visit making replications of longer duration necessary.
Keywords: pharmacological treatment/change; pharmacotherapy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study.Lancet Psychiatry. 2018 Oct;5(10):797-807. doi: 10.1016/S2215-0366(18)30252-9. Epub 2018 Aug 13. Lancet Psychiatry. 2018. PMID: 30115598 Clinical Trial.
-
A randomized double-blind controlled trial to assess the benefits of amisulpride and olanzapine combination treatment versus each monotherapy in acutely ill schizophrenia patients (COMBINE): methods and design.Eur Arch Psychiatry Clin Neurosci. 2020 Feb;270(1):83-94. doi: 10.1007/s00406-019-01063-4. Epub 2019 Sep 5. Eur Arch Psychiatry Clin Neurosci. 2020. PMID: 31486890
-
Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial.Lancet Psychiatry. 2020 Nov;7(11):945-954. doi: 10.1016/S2215-0366(20)30341-2. Lancet Psychiatry. 2020. PMID: 33069317 Clinical Trial.
-
Comparative efficacy and safety between amisulpride and olanzapine in schizophrenia treatment and a cost analysis in China: a systematic review, meta-analysis, and cost-minimization analysis.BMC Psychiatry. 2018 Sep 5;18(1):286. doi: 10.1186/s12888-018-1867-8. BMC Psychiatry. 2018. PMID: 30185173 Free PMC article.
-
Maximizing response to first-line antipsychotics in schizophrenia: a review focused on finding from meta-analysis.Psychopharmacology (Berl). 2019 Feb;236(2):545-559. doi: 10.1007/s00213-018-5133-z. Epub 2018 Nov 30. Psychopharmacology (Berl). 2019. PMID: 30506237 Review.
Cited by
-
Data-Driven Taxonomy for Antipsychotic Medication: A New Classification System.Biol Psychiatry. 2023 Oct 1;94(7):561-568. doi: 10.1016/j.biopsych.2023.04.004. Epub 2023 Apr 14. Biol Psychiatry. 2023. PMID: 37061079 Free PMC article.
-
Switching antipsychotics versus continued current treatment in people with non-responsive schizophrenia.Cochrane Database Syst Rev. 2025 Apr 11;4(4):CD011885. doi: 10.1002/14651858.CD011885.pub2. Cochrane Database Syst Rev. 2025. PMID: 40214650
-
Early Non-Response to Antipsychotic Treatment in Schizophrenia: A Systematic Review and Meta-Analysis of Evidence-Based Management Options.CNS Drugs. 2023 Jun;37(6):499-512. doi: 10.1007/s40263-023-01009-4. Epub 2023 Jun 1. CNS Drugs. 2023. PMID: 37261669
-
Strategies for Switching between Oral Postsynaptic Antidopaminergic Antipsychotics in Patients with Schizophrenia: A Systematic Review.CNS Drugs. 2025 Jul 23. doi: 10.1007/s40263-025-01206-3. Online ahead of print. CNS Drugs. 2025. PMID: 40699529
-
Relationship between efficacy and common metabolic parameters in first-treatment drug-naïve patients with early non-response schizophrenia: a retrospective study.Ann Gen Psychiatry. 2023 Feb 17;22(1):6. doi: 10.1186/s12991-023-00436-3. Ann Gen Psychiatry. 2023. PMID: 36800967 Free PMC article.
References
-
- Lehman AF, Lieberman JA, Dixon LB, et al. . Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2):1–56. - PubMed
-
- Gaebel W, Falkai P, Weinmann S, Wobrock T.. Behandlungsleitlinie Schizophrenie. Darmstadt: Steinkopff; 2006.
-
- Samara MT, Leucht C, Leeflang MM, et al. . Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry. 2015;172(7):617–629. - PubMed
-
- Hatta K, Otachi T, Fujita K, et al. . Antipsychotic switching versus augmentation among early non-responders to risperidone or olanzapine in acute-phase schizophrenia. Schizophr Res. 2014;158(1–3):213–222. - PubMed
-
- Klimke A, Klieser E, Lehmann E, Miele L.. Initial improvement as a criterion for drug choice in acute schizophrenia. Pharmacopsychiatry. 1993;26(1):25–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical