Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity
- PMID: 35857825
- PMCID: PMC9835395
- DOI: 10.1126/scitranslmed.abn1413
Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity
Abstract
To combat the HIV epidemic and emerging threats such as SARS-CoV-2, immunization strategies are needed that elicit protection at mucosal portals of pathogen entry. Immunization directly through airway surfaces is effective in driving mucosal immunity, but poor vaccine uptake across the mucus and epithelial lining is a limitation. The major blood protein albumin is constitutively transcytosed bidirectionally across the airway epithelium through interactions with neonatal Fc receptors (FcRn). Exploiting this biology, here, we demonstrate a strategy of "albumin hitchhiking" to promote mucosal immunity using an intranasal vaccine consisting of protein immunogens modified with an amphiphilic albumin-binding polymer-lipid tail, forming amph-proteins. Amph-proteins persisted in the nasal mucosa of mice and nonhuman primates and exhibited increased uptake into the tissue in an FcRn-dependent manner, leading to enhanced germinal center responses in nasal-associated lymphoid tissue. Intranasal immunization with amph-conjugated HIV Env gp120 or SARS-CoV-2 receptor binding domain (RBD) proteins elicited 100- to 1000-fold higher antigen-specific IgG and IgA titers in the serum, upper and lower respiratory mucosa, and distal genitourinary mucosae of mice compared to unmodified protein. Amph-RBD immunization induced high titers of SARS-CoV-2-neutralizing antibodies in serum, nasal washes, and bronchoalveolar lavage. Furthermore, intranasal amph-protein immunization in rhesus macaques elicited 10-fold higher antigen-specific IgG and IgA responses in the serum and nasal mucosa compared to unmodified protein, supporting the translational potential of this approach. These results suggest that using amph-protein vaccines to deliver antigen across mucosal epithelia is a promising strategy to promote mucosal immunity against HIV, SARS-CoV-2, and other infectious diseases.
Conflict of interest statement
Figures
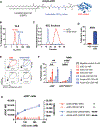
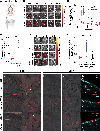


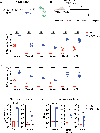
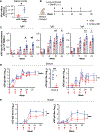
Comment in
-
A hitchhiker's guide to mucosal and systemic immunity.Sci Transl Med. 2022 Jul 20;14(654):eadc8697. doi: 10.1126/scitranslmed.adc8697. Epub 2022 Jul 20. Sci Transl Med. 2022. PMID: 35857822
References
-
- Neutra MR, Kozlowski PA, Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 6, 148–158 (2006). - PubMed
-
- Holmgren J, Czerkinsky C, Mucosal immunity and vaccines. Nat. Med. 11, S45–S53 (2005). - PubMed
-
- Lycke N, Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 12, 592–605 (2012). - PubMed
-
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H, The mucosal immune system: From fundamental concepts to vaccine development. Vaccine 10, 75–88 (1992). - PubMed
-
- Mitragotri S, Immunization without needles. Nat. Rev. Immunol. 5, 905–916 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

