Relayed hyperpolarization for zero-field nuclear magnetic resonance
- PMID: 35857837
- PMCID: PMC9299534
- DOI: 10.1126/sciadv.abp9242
Relayed hyperpolarization for zero-field nuclear magnetic resonance
Abstract
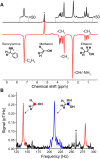
Zero- to ultralow-field nuclear magnetic resonance (ZULF NMR) is a rapidly developing form of spectroscopy that provides rich spectroscopic information in the absence of large magnetic fields. However, signal acquisition still requires a mechanism for generating a bulk magnetic moment for detection, and the currently used methods only apply to a limited pool of chemicals or come at prohibitively high cost. We demonstrate that the parahydrogen-based SABRE (signal amplification by reversible exchange)-Relay method can be used as a more general means of generating hyperpolarized analytes for ZULF NMR by observing zero-field J-spectra of [13C]-methanol, [1-13C]-ethanol, and [2-13C]-ethanol in both 13C-isotopically enriched and natural abundance samples. We explore the magnetic field dependence of the SABRE-Relay efficiency and show the existence of a second maximum at 19.0 ± 0.3 mT. Despite presence of water, SABRE-Relay is used to hyperpolarize ethanol extracted from a store-bought sample of vodka (%PH ~ 0.1%).
Figures

Similar articles
-
Zero- to ultralow-field nuclear magnetic resonance J-spectroscopy with commercial atomic magnetometers.J Magn Reson. 2020 May;314:106723. doi: 10.1016/j.jmr.2020.106723. Epub 2020 Apr 3. J Magn Reson. 2020. PMID: 32298993
-
In Situ SABRE Hyperpolarization with Earth's Field NMR Detection.Molecules. 2019 Nov 14;24(22):4126. doi: 10.3390/molecules24224126. Molecules. 2019. PMID: 31739621 Free PMC article.
-
Hyperpolarization of cis-15 N2 -Azobenzene by Parahydrogen at Ultralow Magnetic Fields*.Chemphyschem. 2021 Jul 16;22(14):1527-1534. doi: 10.1002/cphc.202100160. Epub 2021 Jun 8. Chemphyschem. 2021. PMID: 33932314 Free PMC article.
-
Synthetic Approaches for 15 N-Labeled Hyperpolarized Heterocyclic Molecular Imaging Agents for 15 N NMR Signal Amplification by Reversible Exchange in Microtesla Magnetic Fields.Chemistry. 2021 Jul 7;27(38):9727-9736. doi: 10.1002/chem.202100212. Epub 2021 May 21. Chemistry. 2021. PMID: 33856077 Free PMC article. Review.
-
Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis.Angew Chem Int Ed Engl. 2018 Jun 4;57(23):6742-6753. doi: 10.1002/anie.201710406. Epub 2018 Apr 27. Angew Chem Int Ed Engl. 2018. PMID: 29316071 Review.
Cited by
-
Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange.Chem Sci. 2023 Aug 29;14(36):9843-9853. doi: 10.1039/d3sc03078d. eCollection 2023 Sep 20. Chem Sci. 2023. PMID: 37736655 Free PMC article.
-
Identifying routes for transferring spin polarization from parahydrogen to protic solvents.Chem Commun (Camb). 2024 Nov 21;60(94):13923-13926. doi: 10.1039/d4cc03468f. Chem Commun (Camb). 2024. PMID: 39503617 Free PMC article.
-
Enzymatic Reactions Observed with Zero- and Low-Field Nuclear Magnetic Resonance.Anal Chem. 2023 Dec 12;95(49):17997-18005. doi: 10.1021/acs.analchem.3c02087. Epub 2023 Dec 4. Anal Chem. 2023. PMID: 38047582 Free PMC article.
-
Unconventional Parahydrogen-Induced Hyperpolarization Effects in Chemistry and Catalysis: From Photoreactions to Enzymes.ACS Catal. 2025 Apr 4;15(8):6386-6409. doi: 10.1021/acscatal.4c07870. eCollection 2025 Apr 18. ACS Catal. 2025. PMID: 40270879 Free PMC article. Review.
-
Development of a fully automated workstation for conducting routine SABRE hyperpolarization.Sci Rep. 2024 Sep 9;14(1):21022. doi: 10.1038/s41598-024-71354-x. Sci Rep. 2024. PMID: 39251663 Free PMC article.
References
-
- Giraudeau P., Challenges and perspectives in quantitative NMR. Magn. Reson. Chem. 55, 61–69 (2017). - PubMed
-
- Cavallari E., Carrera C., Aime S., Reineri F., Metabolic studies of tumor cells using [1-13C] pyruvate hyperpolarized by means of PHIP-side arm hydrogenation. ChemPhysChem 20, 318–325 (2019). - PubMed
-
- Basser P. J., Jones D. K., Diffusion-tensor MRI: Theory, experimental design and data analysis—A technical review. NMR Biomed. 15, 456–467 (2002). - PubMed
-
- Damadian R., Goldsmith M., Minkoff L., NMR in cancer: XVI. FONAR image of the live human body. Physiol. Chem. Phys. 9, 97–100 (1977). - PubMed
-
- Eills J., Blanchard J. W., Bougas L., Kozlov M. G., Pines A., Budker D., Measuring molecular parity nonconservation using nuclear-magnetic-resonance spectroscopy. Phys. Rev. A. 96, 042119 (2017).
LinkOut - more resources
Full Text Sources

