More comprehensive proprioceptive stimulation of the hand amplifies its cortical processing
- PMID: 35858122
- PMCID: PMC9423773
- DOI: 10.1152/jn.00485.2021
More comprehensive proprioceptive stimulation of the hand amplifies its cortical processing
Abstract
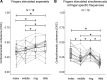
Corticokinematic coherence (CKC) quantifies the phase coupling between limb kinematics and cortical neurophysiological signals reflecting proprioceptive feedback to the primary sensorimotor (SM1) cortex. We studied whether the CKC strength or cortical source location differs between proprioceptive stimulation (i.e., actuator-evoked movements) of right-hand digits (index, middle, ring, and little). Twenty-one volunteers participated in magnetoencephalography measurements during which three conditions were tested: 1) simultaneous stimulation of all four fingers at the same frequency, 2) stimulation of each finger separately at the same frequency, and 3) simultaneous stimulation of the fingers at finger-specific frequencies. CKC was computed between MEG responses and accelerations of the fingers recorded with three-axis accelerometers. CKC was stronger (P < 0.003) for the simultaneous (0.52 ± 0.02) than separate (0.45 ± 0.02) stimulation at the same frequency. Furthermore, CKC was weaker (P < 0.03) for the simultaneous stimulation at the finger-specific frequencies (0.38 ± 0.02) than for the separate stimulation. CKC source locations of the fingers were concentrated in the hand region of the SM1 cortex and did not follow consistent finger-specific somatotopic order. Our results indicate that proprioceptive afference from the fingers is processed in partly overlapping cortical neuronal circuits, which was demonstrated by the modulation of the finger-specific CKC strengths due to proprioceptive afference arising from simultaneous stimulation of the other fingers of the same hand as well as overlapping cortical source locations. Finally, comprehensive simultaneous proprioceptive stimulation of the hand would optimize functional cortical mapping to pinpoint the hand region, e.g., prior brain surgery.NEW & NOTEWORTHY Corticokinematic coherence (CKC) can be used to study cortical proprioceptive processing and localize proprioceptive hand representation. Our results indicate that proprioceptive stimulation delivered simultaneously at the same frequency to fingers (D2-D4) maximizes CKC strength allowing robust and fast localization of the human hand region in the sensorimotor cortex using MEG.
Keywords: acceleration; corticokinematic coherence; magnetoencephalography; proprioception; sensorimotor cortex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Corticokinematic coherence is stronger to regular than irregular proprioceptive stimulation of the hand.J Neurophysiol. 2021 Aug 1;126(2):550-560. doi: 10.1152/jn.00095.2021. Epub 2021 Jul 14. J Neurophysiol. 2021. PMID: 34259024
-
Proprioceptive response strength in the primary sensorimotor cortex is invariant to the range of finger movement.Neuroimage. 2023 Apr 1;269:119937. doi: 10.1016/j.neuroimage.2023.119937. Epub 2023 Feb 13. Neuroimage. 2023. PMID: 36791896
-
Volitional muscle activation intensifies neuronal processing of proprioceptive afference in the primary sensorimotor cortex: an EEG study.J Neurophysiol. 2024 Jan 1;131(1):28-37. doi: 10.1152/jn.00340.2023. Epub 2023 Nov 15. J Neurophysiol. 2024. PMID: 37964731
-
Reproducibility of corticokinematic coherence.Neuroimage. 2018 Oct 1;179:596-603. doi: 10.1016/j.neuroimage.2018.06.078. Epub 2018 Jun 30. Neuroimage. 2018. PMID: 29964185
-
Coupling between human brain activity and body movements: Insights from non-invasive electromagnetic recordings.Neuroimage. 2019 Dec;203:116177. doi: 10.1016/j.neuroimage.2019.116177. Epub 2019 Sep 9. Neuroimage. 2019. PMID: 31513941 Review.
Cited by
-
Identification of proprioceptive thalamocortical tracts in children: comparison of fMRI, MEG, and manual seeding of probabilistic tractography.Cereb Cortex. 2022 Aug 22;32(17):3736-3751. doi: 10.1093/cercor/bhab444. Cereb Cortex. 2022. PMID: 35040948 Free PMC article.
-
Reproducibility of evoked and induced MEG responses to proprioceptive stimulation of the ankle joint.Neuroimage Rep. 2022 Jun 13;2(3):100110. doi: 10.1016/j.ynirp.2022.100110. eCollection 2022 Sep. Neuroimage Rep. 2022. PMID: 40567313 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

