Open-Label, Randomized, Multicenter, Phase III Study Comparing Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer
- PMID: 35858154
- PMCID: PMC9788977
- DOI: 10.1200/JCO.21.02953
Open-Label, Randomized, Multicenter, Phase III Study Comparing Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer
Abstract
Purpose: Intravenous paclitaxel (IVpac) is complicated by neuropathy and requires premedication to prevent hypersensitivity-type reactions. Paclitaxel is poorly absorbed orally; encequidar (E), a novel P-glycoprotein pump inhibitor, allows oral absorption.
Methods: A phase III open-label study comparing oral paclitaxel plus E (oPac + E) 205 mg/m2 paclitaxel plus 15 mg E methanesulfonate monohydrate 3 consecutive days per week versus IVpac 175 mg/m2 once every 3 weeks was performed. Women with metastatic breast cancer and adequate organ function, at least 1 year from last taxane, were randomly assigned 2:1 to oPac + E versus IVpac. The primary end point was confirmed radiographic response using RECIST 1.1, assessed by blinded independent central review. Secondary end points included progression-free survival (PFS) and overall survival (OS).
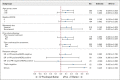
Results: Four hundred two patients from Latin America were enrolled (265 oPac + E, 137 IVpac); demographics and prior therapies were balanced. The confirmed response (intent-to-treat) was 36% for oPac + E versus 23% for IVpac (P = .01). The PFS was 8.4 versus 7.4 months, respectively (hazard ratio, 0.768; 95.5% CI, 0.584 to 1.01; P = .046), and the OS was 22.7 versus 16.5 months, respectively (hazard ratio, 0.794; 95.5% CI, 0.607 to 1.037; P = .08). Grade 3-4 adverse reactions were 55% with oPac + E and 53% with IVpac. oPac + E had lower incidence and severity of neuropathy (2% v 15% > grade 2) and alopecia (49% v 62% all grades) than IVpac but more nausea, vomiting, diarrhea, and neutropenic complications, particularly in patients with elevated liver enzymes. On-study deaths (8% oPac + E v 9% IVpac) were treatment-related in 3% and 0%, respectively.
Conclusion: oPac + E increased the confirmed tumor response versus IVpac, with trends in PFS and OS. Neuropathy was less frequent and severe with oPac + E; neutropenic serious infections were increased. Elevated liver enzymes at baseline predispose oPac + E patients to early neutropenia and serious infections (funded by Athenex, Inc; ClinicalTrials.gov identifier: NCT02594371).
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

Comment in
-
Assessing the Clinical Utility of Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer.J Clin Oncol. 2023 Feb 20;41(6):1323. doi: 10.1200/JCO.22.01759. Epub 2022 Nov 3. J Clin Oncol. 2023. PMID: 36331242 No abstract available.
References
-
- Panday VRN, Huizing MT, Ten Bokkel Huinink WW, et al. : Hypersensitivity reactions to the taxanes paclitaxel and docetaxel. Clin Drug Invest 14:418-427, 1997
-
- Seidman AD, Berry D, Cirrincione C, et al. : Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642-1649, 2008 - PubMed

